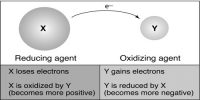
Oxidation number
Positive or negative charge number in the cation and anion produced in the reactions of the related elements is called oxidation number.
Rules for determining oxidation number of an element: The following general rules are used to determine the oxidation number of elements:
- The oxidation number of an element in free or elementary state is always For e.g. in the reaction of hydrogen and oxygen in the formation of water is the oxidation number of both hydrogen and oxygen is zero.
2H2 + O2 –> 2H2O - The oxidation number of an element in a single (mono atomic) ion is the same as the charge on the ion. For e.g. Oxidation number of K+ is +1.
- In binary compounds of metal and non-metals the oxidation of metal is always positive while that of the non-metal is negative. E.g. in NaCl the oxidation number of Na is +1 and the oxidation number of Cl is -1.
- In compounds formed by the combination of non-metallic atoms the atoms with higher electro-negativity is given negative oxidation number. For e.g. in HCl the oxidation number of Cl is -1 because of its higher electro-negativity.
- In all compound of hydrogen, the oxidation number of hydrogen is always H except in Hydrides of active metals such as LiH, NaH, KH, MgH2, CaH2, etc. Where Hydrogen has the oxidation number of -1.
- In compounds containing oxygen, the oxidation number of oxygen is -2 except in peroxide, such as Na2O2 and in OF2. The oxidation of oxygen in peroxides (Na2O2) is -1 and in OF2 its oxidation number is +2.
- For neutral molecule the sum of the oxidation number of all the atoms is equal to zero. e.g. In NH3 the sum of the oxidation numbers of nitrogen atom and 3 Hydrogen atoms is equal to zero. For a complex ion, the sum of the oxidation numbers of the atoms is equal to charge of the ion. e.g. In SO42- ion, the sum of the oxidation of sulphur atom and 4 oxygen atoms must be equal to -2.
- Oxidation number of the following elements is always fixed:
- The oxidation number of group IA metallic like Li, Na, K, Rb and Cs is always +1.
- Oxidation number of group IIA metals like Be, Mg, Ca, Sr, Ba is always +2.
- Oxidation number of Al is always +3.
- Oxidation number of F is always -1.