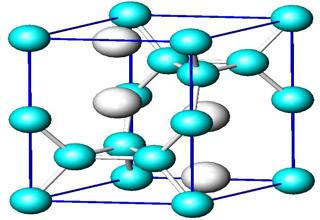
An X-ray analysis shows that the ions in solids are arranged in different patterns. The exact arrangement of the ions in an ionic lattice varies according to the particular ions. In an ionic lattice the positive and negative ions may have similar sizes or may be quite different in size. The relative sizes of the ions influence the numbers which can pack together and this dictates the geometry of the crystal.
In mineralogy and crystallography, a crystal structure is a unique arrangement of atoms in a crystal. A crystal structure is composed of a unit cell, a set of atoms arranged in a particular way; which is periodically repeated in three dimensions on a lattice.
Using X-Ray crystallography
The ions in an ionic crystal lattice are closely packed together in a basic repeating building block called a Unit cell. The Unit cell, determined by X-ray crystallography, contains all the structural information about a crystal.
The whole crystal (in theory) could be constructed from sticking lots of these units together. A particular arrangement of atoms in a crystal structure can be described by specifying the atom positions in a repeating “unit cell”










