Decaborane, also known as decaborane(14), is a borane with the chemical formula B10H14. It is one of the most important boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. This white crystalline compound is one of the most important boron hydride clusters, serving as both a reference structure and a precursor to other boron hydrides. It is toxic and volatile, with a foul odor.
Although it has been extensively researched, it has no significant applications. Decaborane is potentially useful as a fuel for aneutronic fusion and for low energy ion implantation of boron in the fabrication of semiconductors because it decomposes in a plasma, yielding monoatomic boron ions. It has also been considered for plasma-assisted chemical vapor deposition for the manufacture boron-containing thin films.
Properties
- Chemical formula: B10H14
- Molar mass: 122.22 g/mol
- Appearance: White crystals
- Odor: bitter, chocolate-like
- Density: 0.94 g/cm3
- Melting point: 97–98 °C (207–208 °F; 370–371 K)
- Boiling point: 213 °C (415 °F; 486 K)
- Solubility in other solvents: Slightly, in cold water.
- Vapor pressure: 0.2 mmHg

Handling and structure
The physical properties of decaborane(14) are similar to those of naphthalene and anthracene, which are all volatile colorless solids. The most common method of purification is sublimation. Decaborane is highly flammable, but it burns with a bright green flame, like other boron hydrides. It is not sensitive to moist air, but it hydrolyzes in boiling water, releasing hydrogen and producing a boric acid solution. It is soluble in cold water and a number of non-polar and moderately polar solvents.
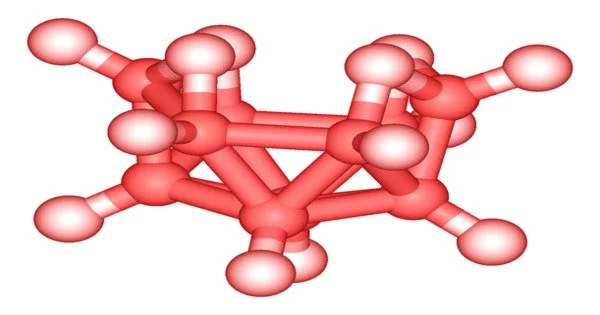
The B10 framework in decaborane resembles an incomplete octadecahedron. Each boron has one “radial” hydride, and four boron atoms near the cluster’s open end have additional hydrides. The structure is referred to as a “nido” in cluster chemistry.
Synthesis and reactions
It is commonly synthesized via the pyrolysis of smaller boron hydride clusters. For example, pyrolysis of B2H6 or B5H9 gives decaborane, with loss of H2. On a laboratory scale, sodium borohydride is treated with boron trifluoride to give NaB11H14, which is acidified to release borane and hydrogen gas.
It reacts with Lewis bases (L) such as CH3CN and Et2S, to form adducts:
B10H14 + 2 L → B10H12L2 + H2
These species, which are classified as “arachno” clusters, in turn react with acetylene to give the “closo” ortho-carborane:
B10H12·2L + C2H2 → C2B10H12 + 2 L + H2
Decaborane(14) is a weak Brønsted acid. Monodeprotonation generates the anion [B10H13]−, with again a nido structure.
In the Brellochs reaction, decaborane is converted to arachno-CB9H14−:
B10H14 + CH2O + 2 OH− + H2O → CB9H14− + B(OH)4− + H2
Applications
Decaborane has no significant applications, despite the fact that it has been extensively researched.
LPP Fusion announced plans to use decaborane in its next round of fusion experiments in 2018. Decaborane has been evaluated for low energy ion implantation of boron in semiconductor manufacturing. It’s also been considered for plasma-assisted chemical vapor deposition to make boron-containing thin films.
Decaborane is a good reagent for reductive amination of ketones and aldehydes.
Safety
Decaborane, like pentaborane, is a powerful toxin that affects the central nervous system, though it is less toxic. It is absorbed through the skin.
Sublimation purification necessitates the use of a dynamic vacuum to remove evolved gases. Near 100 °C, crude samples explode. It reacts explosively with carbon tetrachloride, resulting in an explosion in a manufacturing facility.