Diffusion: At normal temperature and pressure, when molecules of any substance of it’s area of higher concentration diffuse to the area of lower concentration is said to be diffusion.
Diffusion may take the plate in solid, liquid and gaseous substances but it must occur from the area of higher concentration to the area of lower concentration. Diffusion will be stopped when the concentration of the substances becomes equal. The rate of diffusion increases as the temperature increases. At higher temperatures, particles move faster because more energy is available to diffuse them.
Examples:
- If potassium permanganate powder is put in a glass of water potassium permanganate powder will diffuse into the water and the color of the water will turn into violet. It’s a diffusion method.
- If a few drops of scent is taken from the bottle and used in human body its smell will spread all over the room. This is also a diffusion method.
Diffusion regulatory factors:
Temperature: When temperature increases the rate of diffusion will also Increase, because of the movement of the participating molecules is increased due to the increase in temperature.
The density of the substances: Concentration of the substances which is involved in diffusion is proportional to the rate of diffusion; example: less concentrated substance decreases the rate of diffusion.
Atmospheric pressure: Atmospheric pressure is inversely proportional to the rate of diffusion.
Importance of diffusion:
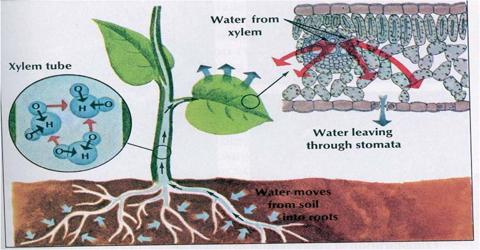
- Atmospheric carbon dioxide diffuses to leave during photosynthesis.
- Atmospheric oxygen diffuses to cells during respiration.
- Excessive water comes out of the plan body during transpiration.
- Absorption of mineral salts occurs due to diffusion