Chemical reactions of Alkane:
Substitution reaction: It is the substitution reaction of alkane with Chlorine (Cl2), Bromine (Br2) etc.
- Halogenation of alkane: It takes place in the presence of diffused sunlight, UV rays or in high temperature (3000 — 4000 C), yielding products are CH2CI2, CHC13, CCl4
- The energy of C—H bond is 413 kjmol-1.
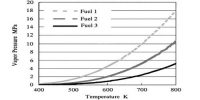
Pyrolysis/Cracking/Thermal Dissociation: It takes place in high temperature (500 – 8000 C) and in the absence of air.
- More volatile alkane can he prepared by cracking
- What type of product will be yielded at decomposition, it depends on nature, temperature and pressure of Alkane and the Catalyst.
Isomerism of Alkane
- It takes place in the presence of dry AlC13 and HCI gas mixture.
- Branched alkanes are good fuel.