We know that the charge of one electron is 1.60 x 10-19 C. Thus, the charge carried by one mole of electrons will be 6.02 x 1023 x 1.60 x 10-19 = 96,500 coulombs.
We know that 96,500 C is also called 1 Faraday (named after the English scientist Michael Faraday). The Faraday constant (F) is the amount of charge in one mole of electrons. A mole of electrons is given a special name: 1 Faraday. The charge of 1 mole of electrons is called the Faraday Constant, F.
In moving one mol of electrons through a circuit, the value of the work done by an electro-chemical cell is the product of the Faraday constant (referred to as F) by the potential difference between the electrodes as shown below:
Work [J] = – F [C] * Potential Difference [V]
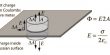
We can now write an expression for the maximum work attainable by an electro-chemical cell. Let “n” be the number of electrons (in mol) transferred in the overall electrochemical cell reaction. The maximum work (Wmax) for molar amounts of reactants is given by the following formula:
Wmax =-n x F x Ecell