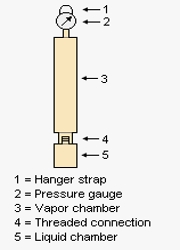
There is of course no such thing as ‘the vapour pressure’ of a gasoline. This is because the observed vapour pressure depends upon the volume into which the vapour expands. The standard industry measure is the Reid Vapour Pressure, where the volume occupied by the vapour is the same between tests, hopefully enabling at least comparative differences between gasolines to be measured. The RVP, which is measured at 38°C, is sometimes determined for crude oil and for other petroleum fractions including kerosene. A synthesis of selected recent literature appertaining to the RVP of gasolines and blends containing gasoline follows in tabular form.
Trends evident from the limited but certainly representative entries in the table are that ethanol raises the RVP and ETBE lowers it. ETBE is one of a number of oxygenated additives for gasoline. Methyl tertiary butyl ether (MTBE) is another, and it has been observed that this causes a rise in the RVP as does methanol when that is blended with gasoline.
FCC gasoline tends to have somewhat lower values of the RVP than a ‘base gasoline The vapour pressure of iso-octane at 38°C is 14 kPa, suggesting that for vapour pressure purposes iso-octane is not the best reference hydrocarbon compound. Cyclopentane has a vapour pressure of 68 kPa at 38°C and is thus a good benchmark for ‘winter gasoline’.