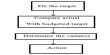
Dissociation enthalpy of weak base Ammonium hydroxide (NH4OH)
Let consider,
Strong Acid, HCl = H+ + Cl– (Completely)
Strong base, NaOH = Na+ + OH– (Completely)
Weak base, NH4OH = NH4+ + OH– — energy (completely)
or, NH4OH + energy = NH4+ + OH–
The energy is used for dissociation because of NH4OH is weak base. This energy is used from solution. The dissociation enthalpy of NH4OH = (57.32 — 51.46) Kj mol-1 = 5.86 Kj mol-1