The olfactory mucosa is made up of the olfactory epithelium and the underlying lamina propria, connective tissue containing fibroblasts, blood vessels, Bowman’s glands, and bundles of fine axons from the olfactory neurons. It is located in the upper section of the nasal cavity. The mucus protects the olfactory epithelium while also allowing scents to dissolve and be recognized by olfactory receptor neurons. Bowman’s glands include cells with enormous secretory vesicles, according to electron microscopy research. The specific makeup of Bowman’s gland secretions is unknown, but there is evidence that they generate odorant binding protein.
Olfaction is controlled by olfactory mucosal cells, which are found in the upper nasal cavity. Although olfactory impairment appears early in several neurodegenerative illnesses, including Alzheimer’s disease, disease-related modifications to the olfactory mucosal cells are poorly understood.
Researchers at the University of Eastern Finland created and defined a new cell model for Alzheimer’s disease that has broad scientific applications and could aid in early detection and testing of new medicines.
This novel technique has showed that cells in the nasal cavity are defective in Alzheimer’s disease patients. Not only are transcriptional changes observed, but the functionality of these patient-derived cells are also compromised.
Riikka Lampinen
The olfactory mucosa (OM) is essential for smell and is in direct touch with the brain. The OM is made up of three major components: the epithelium, the basement membrane, and the lamina propria. The healthy human OM in vivo is made up of a variety of cell types, including olfactory sensory neurons, basal cells (stem cells), Bowman’s gland cells, vascular smooth muscle cells, sustentacular cells, and immunological cells. Human OM cells can be acquired for research purposes through a very non-invasive biopsy of the nasal septum performed under local anesthesia.
Over several years, the researchers collected nasal biopsies from cognitively healthy individuals and patients diagnosed with Alzheimer’s disease in collaboration with clinicians at Kuopio University Hospital. After processing the tissue, the researchers used a sophisticated single cell RNA sequencing technique to describe the patient-derived cells of the olfactory mucosa, which are found in the upper portions of the nasal canal. The olfactory mucosal tissue is important for the sensation of smell because it contains olfactory receptor neurons that extend to the brain.
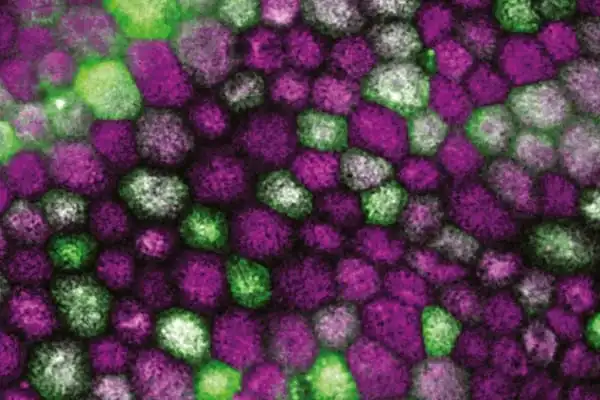
“This novel technique has showed that cells in the nasal cavity are defective in Alzheimer’s disease patients,” explains Riikka Lampinen, an early-stage researcher. “Not only are transcriptional changes observed, but the functionality of these patient-derived cells are also compromised.” The novel cell model and its findings were published today in Cells.
Alzheimer’s disease is a terrible, chronic disease for which there is no cure or effective treatment. As a result, new human-centered approaches to understanding and combating disease pathophysiology are required. The new findings from Associate Professor Katja Kanninen’s research group could provide crucial insight into why a substantial proportion of Alzheimer’s disease patients have a damaged sense of smell early in the disease pathogenesis in the future. Furthermore, this unique study model that resembles human physiology has broad utility in permitting deep investigation of disease pathways, which could lead to new therapeutic discoveries.
Anosmia, or loss of smell, has been linked to the early stages of various neurodegenerative disorders, including Alzheimer’s (AD). Patients with late-onset Alzheimer’s disease (AD) had higher levels of amyloid-beta (Aβ) and phosphorylated tau in their nasal secretions, indicating pathogenic processes in the nasal cavity. An immunostaining for filamentous tau in cells proximal to the OM basal membrane was discovered in postmortem histological investigation of the AD OM.
The olfactory mucosa has been hypothesized as a route for air contaminants and viruses to enter the brain. “Our present work is focused on understanding in detail how viral infections or air pollution exposure influence the cells of the olfactory mucosa,” Katja Kanninen explains.
While these findings have yet to be published, they are anticipated to provide vital insight into how substances absorbed in air could get access to the brain and disrupt its function.