Dew point
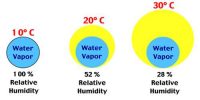
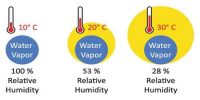
Atmospheric air contains water vapour or moisture. A fixed volume of air can contain a fixed amount of water vapour at a particular temperature. The capacity to contain water vapour increases with increasing temperature and decreases with decreasing temperature. Ordinarily, the atmospheric air is not saturated with water vapour present in it. If for some reason the atmosphere is cooled, it may become saturated with the water vapour present in it. Further cooling of air causes the excess pressure to condense in the form of small droplets. The temperature at which this occurs is called the dew point. Now we will discuss in detail what dew point means.
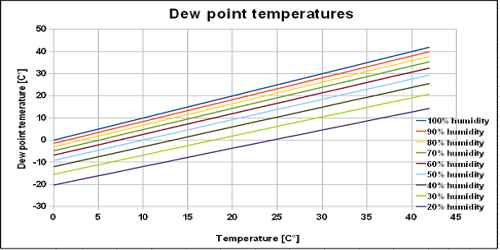
Definition: The temperature at which a given mass of air becomes just saturated by water vapour present in it is called its dew point.
“The dew point at a place is 15°C”- This statement means that at 15°C the air at that place will be saturated with water vapour present in it. It is the temperature at which air is saturated with water vapor, which is the gaseous state of water.
When air has reached the dew-point temperature at a particular pressure, the water vapor in the air is in equilibrium with liquid water, meaning water vapor is condensing at the same rate at which liquid water is evaporating.
The amount of water vapour present in a fixed volume of air at a particular temperature becomes saturated with the same amount of water vapour present in it at dew point. According to Dalton’s law the pressure of the saturated vapour does not depend on the pressure of the air. So, the vapour pressure of air of a fixed volume air at a particular temperature is equal to the saturated vapour pressure at dew point.