Difference between Diffusion and Osmosis:
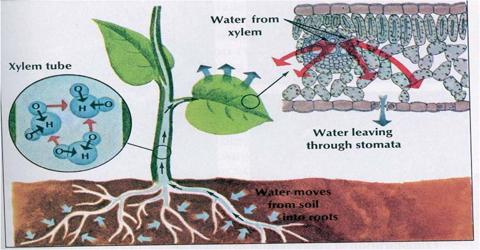
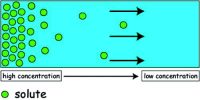
Diffusion is a spontaneous movement of particles from an area of high concentration to an area of low concentration.
- The molecules of the substance are not to pass through the differentially permeable membrane.
- In this process, the molecules of the substance diffuse from the area where they are in high concentration to the area where they are in a low concentration.
- Diffusion may also occur in between two solutions of different natures.
- Diffusion causes diffusion pressure.
- Diffusion may occur in between gas and gas; liquid and gas; solid and liquid; solid and gas.
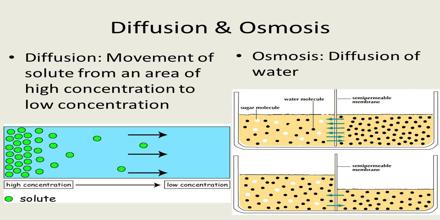
Osmosis is the spontaneous net movement of water across a semipermeable membrane from a region of low solute concentration to a more concentrated solution, up to a concentration gradient.
- The molecules of the solvent are to pass through the differentially permeable membrane.
- In this method, the water molecules diffuse from lower concentrated solution to the higher concentrated solution.
- Osmosis can only occur in between two solutions of same characteristics.
- Osmosis causes osmotic pressure.
- Oman can only occur in between liquid and liquid.