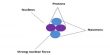
At constant temperature, the dissociation of Phosphorus Pentachloride decreases with increasing pressure.
The reaction: PCl5↔ PCl3 + CO2
no. of moles → 1 1 1
At constant temperature, the effect of pressure change depends on a number of moles of reactant and product. In this reaction the number of moles produces is higher than reactant. For that, the dissociation decreases with increasing pressure.