Effect of catalyst on equilibrium
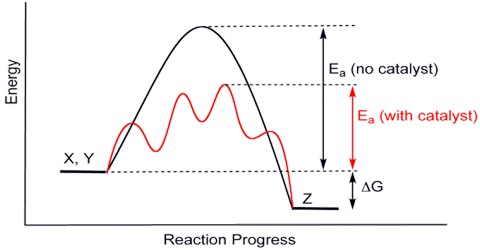
A catalyst does not affect the position of equilibrium and hence it does not have any effect on the value of equilibrium constant of a reaction. This is because a catalyst affects the forward and reverse reaction equally. Catalysts work by producing an alternative route for the reaction. This affects the forward and back reactions equally.
Equilibrium constants are not altered if you insert (or change) a catalyst. The only thing that changes equilibrium constant is an alter of temperature. The situation of equilibrium is not altered if you insert (or change) a catalyst. A catalyst speeds up both the forward and back reactions by exactly the same amount. Dynamic equilibrium is conventional when the rates of the forward and back reactions become equivalent. If a catalyst speeds up both reactions to the similar degree, then they will continue equivalent without any need for a change in position of equilibrium.
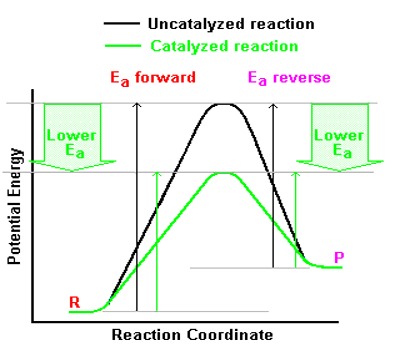
Adding a catalyst makes completely no differentiation to the place of equilibrium, and Le Châtelier’s principle does not affect. This is for the reason that a catalyst speeds up the forward and back reaction to the similar degree and adding a catalyst does not involve the comparative rates of the two reactions, it cannot affect the position of equilibrium.