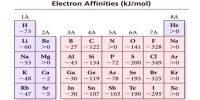
Effect of pressure on solubility:
Pressure: There is no effect of temperature on solubility of solid or liquid solute in a liquid solvent. But if the solute is gas in a liquid solvent then the solubility of gas is increased when the pressure is increased. And if the pressure is decreased then the solubility of gas is decreased. It can be expressed by Henry’s law.
Henry’s law: At a constant temperature the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the pressure (partial) of that gas in equilibrium with that liquid.
P = KHC
Where, p = Partial pressure (atm)
c = Concentration of dissolved gas in liquid (mol) L
k = Tempel azure based constant (L.atm/mol)
Example: At temperature -1°c and the pressure is p then if xg gas is dissolved in 100 liquid. Then in same temperature if the pressure is 4p then the amount of dissolved gas in 100 mL liquid will be 4x g.