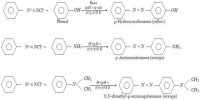
The electrical properties of any hydrocarbon have direct relevance to fire safety. If in movement or in tank filling a hydrocarbon liquid is splashed, static electricity will be generated. No hydrocarbon liquid is a ‘conductor’, but the lower the resistance to conduction of any static so generated the better as such static can become an ignition source for vapours.
The resistivity of pure water at 20°C is 18.6 megaohm cm. The conductivity is the reciprocal of the resistivity, units ohm-1 cm-1 or S cm-1 where S denotes the unit Siemen which has replaced the old ‘reciprocal ohm’ (mho). Hence the conductivity of pure water is:
The final figure in the above row can be re-expressed 5.4 S μm-1. As an example of an organic liquid, ethylbenzene has a value of 123 μS m-1, many orders of magnitude lower. Even though there are very few ions in ‘pure’ water, the polar structure of the molecules provides for some enhancement of electrical conductivity.
Another electrical property of interest in hydrocarbon technology is the capacitance. The capacitance of a dielectric is the amount of charge it can hold per unit potential difference across it. The units are thus:
coulomb/ volts = farad