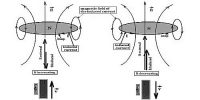
Electromagnetic radiation is an energy that is transmitted through free space or through any element in the form of electronic magnetic waves such as radio waves, visible light, and gamma rays. These include radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays. In such waves, the time-alternating electric and magnetic fields are perpendicular to each other at right angles and perpendicular to the direction of motion. An electromagnetic wave is characterized by its intensity and the frequency of change of electrical and magnetic fields over time. Sunlight is also a form of EM energy, but visible light is only a small part of the EM spectrum, containing a wide range of electromagnetic wavelengths.
Classically, electromagnetic radiation consists of electromagnetic waves that are synchronized oscillations of electric and magnetic fields. In a vacuum, electromagnetic waves travel at the speed of light, usually marked c. In homogeneous, isotropic media, the oscillations of two fields are perpendicular to each other and perpendicular to the direction of energy and wave propagation, forming a transverse wave. In 1864 he introduced his electromagnetic theory and proposed that light – including various forms of radioactive energy – an electromagnetic interference in the form of waves. In 1887, Heinrich Hertz, a German physicist, produced the first man-made electromagnetic waves and provided experimental confirmation by investigating their properties. Scientists are unsure of how waves and particles relate to each other. Electromagnetic radiation travels in waves that allow us to measure a variety of wavelengths or how long the waves are. Electromagnetic waves of different frequencies are called different because they have different sources and effects. For the sequences of increasing frequency and decreasing wavelength, these are radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
A vacuum is the only perfectly transparent medium, and all material media strongly absorb certain regions in the electromagnetic spectrum. For example, molecular oxygen (O2), ozone (O3) and molecular nitrogen (N2) in the Earth’s atmosphere are almost completely transparent compared to infrared rays of all frequencies, but they strongly absorb ultraviolet light, X-rays and gamma rays. EM waves carry energy, speed, and angular momentum away from their source particles and can transmit the amount they interact with. Electromagnetic radiation is related to EM waves that are free to propagate themselves without the uninterrupted effect of their productive running charge (“radiation”), because they have achieved a sufficient distance from these charges. The highest point of a wave is known as ‘crest’, but the lowest point is known as ‘hole’. Electromagnetic waves can be divided into different frequencies. This is known as the electromagnetic spectrum. Moreover, X-ray absorption by a molecular system can cause chemical activity. For example, when X-rays are absorbed into a gas, they expel photoelectrons from the gas, causing its molecules to ionize. This relationship is given by Planck’s equation E = HF, where E is the energy per photon, F is the frequency of the photon, and H is Planck’s constant. For example, a single gamma-ray photon can carry ~ 100,000 times the energy of a single photon of visible light.
There are many sources of natural and man-made, electromagnetic radiation. The effects of EMR on chemical compounds and organic organisms depend on both the energy of the radiation and its frequency. EMR of visible or low frequencies is called non-ionizing radiation because its photons do not have enough energy to individually ionize atoms or molecules or break chemical bonds. For example, radio waves are produced by cosmic objects and electronic circuits such as pulsars and quarries. Sources of ultraviolet radiation include mercury vapor lamps and high-intensity light, as well as the sun. The main features and behavior of electromagnetic radiation are discussed in this article, as it has various forms including its source, individual properties, and practical applications. The article also marks the development of both classical and quantum theories of radiation. These radiations have the ability to cause chemical reactions and damage to living cells as a result of general heat and can be a health hazard.