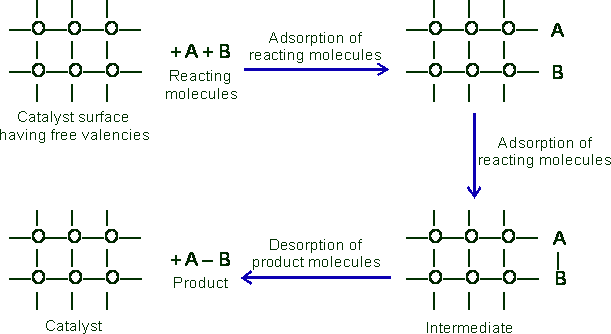
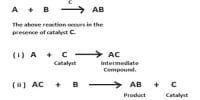
Experiment Explanation by Catalytic Adsorption Theory
The adsorption theory satisfactorily explains the following:
(i) The specific nature of the catalyst and the change in the course of reaction by changing the surface: When the surface material is changed the nature of chemisorptions of the molecules is changed and different reaction products are expected. When ethyl alcohol is decomposed on Al2O3 surface, the principal products are C2H4 and H2O a case of dehydration. In the presence of copper the principal products are acetaldehyde and hydrogen, a case of dehydrogenation. Fe or Pt is specific in their action as catalyst in the synthesis of ammonia.
(ii) The action of catalytic poisons: These substances are strongly adsorbed on the catalyst surface- more so on the active sites, and thus the surface is made unavailable for chemisorptions of the reacting gas. The catalytic activity is, therefore, lost. In temporary poisoning the adsorbed poison can be removed and the surface cleaned rather easily and the catalytic activity regained. But in many cases the poison forms surface compounds with the catalyst and forms, a strong adhering film rendering the catalyst completely inactive. Thus only a small quantity of the poison, sufficient to form a unimolecular layer of “surface compounds” could render a catalyst inactive.