Cleaning mechanism of toilet cleaner.
Caustic soda reacts with fat and oil and form glycerin and soap.
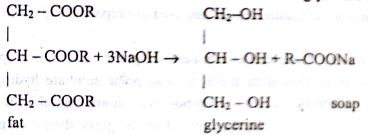
Produced soap and liquid soap used in the preparation of toilet cleaner are the main cleaning agent. Soap molecule has two parts polar and non-polar. Polar carboxylate part are hydrophilic and soluble in water. Non-polar lipophobic parts are soluble in oil & fat.
CH3 — (CH2)16 — COO– Na+
non-polar part polar part
The long chain hydrocarbon parts soluble in the layer of oil and fats on tiles or floor and carboxylate ion are soluble in water. The hydrocarbon parts of soap penetrate into the oil or fat particles. That is why when the toilet is brushed with toilet cleaner the fats or oil become soluble and water remove these. Then the toilet becomes clean, bright and smooth.