Extraction of Zinc
Zinc blende is concentrated by floath floatation process. The pulverized ore is kept in large tank containing water and pine oil. The mixture is agitated by passing compressed air. Ore forms froth and comes to the surface while impurities are left in water. Zinc Blende does not contain a very high percentage of zinc and hence it needs to be concentrated. The best concentration method for zinc ore is known as froth flotation.
Zn is extracted from Zinc blend mainly by 2 ways:
- Carbon Reduction process.
- Electrolysis.
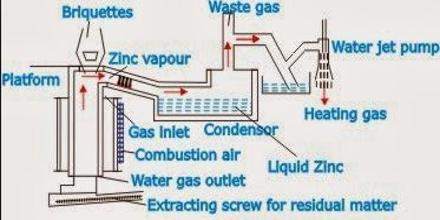
In Carbon reduction process, 04 steps are involved. These are –
a) Concentration: by oil-froth floatation process.
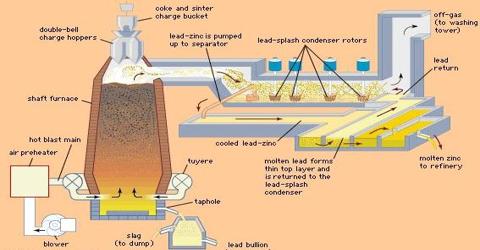
b) Roasting: The concentrated ore is roasted in a current of air in a rotating shaft furnace. The temp of the furnace shouldn’t exceed 900°C
c) Smelting: It is done in a number of cylindrical fireclay retorts. The mixture of ZnO & coke is heated at I3500C for 24 hours in the gas burner. The reactions in the retort are-
- ZnO + C → 500C→ ZnO + CO
- ZnO + CO → ZnO + CO2
- CO2 + C → 2CO
Zinc, thus obtained is 97-98% pure & is called Spelter or Commercial Zinc. Speller can be further purified by electrolysis.
Uses of Zinc:
- Zinc is a metal. It is called an “essential trace element” because very small amounts of zinc are necessary for human health.
- It is used for treatment and prevention of zinc deficiency
- Zinc sulfate is used in products for eye irritation.