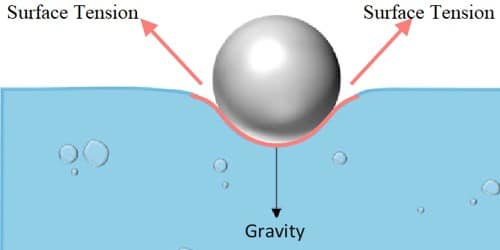
Impurities present in a liquid appreciably affect surface tension. A highly soluble substance like salt increases the surface tension whereas sparingly soluble substances like soap decrease the surface tension.
The surface tension decreases with rising in temperature. The temperature at which the surface tension of a liquid becomes zero is called the critical temperature of the liquid. Oxygen in the atmosphere is known to decrease the surface tension of various substances.
Key points of Factors affecting surface tension:
- Temperature ↑ surface tension ↓
- Critical temperature Surface tension: Zero
- The critical temperature of water 3744K
- Increase with impurity.
Let’s consider the effects of these four conditions on surface tension:
- Temperature
As temperature decreases, surface tension increases. The further the temperature increases we can say surface tension decreases. On the other hand, as surface tension decreases strongly; as molecules become more dynamic with an increase in temperature becoming zero at its boiling point and desertion at decisive temperature.
- Chemical Additions
Adding chemicals to a liquid will transform its surface tension characteristics. You can transform surface tension by adding further chemicals. The consequence of adding an unrelated chemical to a material, and thereby changing its surface tension, is confirmed by the example of putting soap (a surfactant) in water to decrease the surface tension, which allows the dirt on your hands to more simply mix with the water. The examples which our teachers used to give us includes accumulation soap to water
- Oxidation
Oxidation straight affects surface tension. When surface tension increases, intermolecular forces increase. As surface tension increases, intermolecular forces increase. Oxygen in the atmosphere is known to reduce the surface tension of a variety of substances.
- The Presence of Impurities
The presence of impurities on the surface of, or dissolved in, a material straight affects the surface tension of the liquid. The surface tension of water, for example, will increase when extremely soluble impurities are added to it.
- Surfactant
Now that we’ve measured the effects of dissimilarity in temperature, the addition of chemicals, oxidation, and the incidence of impurities on surface tension, perhaps we should subsequently think about the effect of a surfactant over time and how we monitor and determine this effect.