The half-life of a Radioactive Element
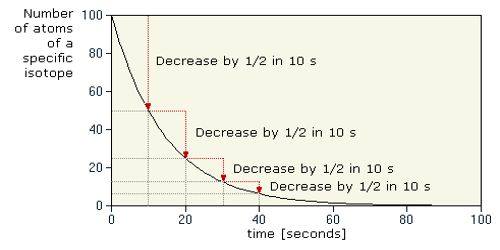
The half-life of a radioactive element is defined as the time during which the number of atoms remaining unchanged becomes half of its initial value. It measures the time it takes for a given amount of the substance to become reduced by half as a consequence of decay, and therefore, the emission of radiation.
From the law of radioactive disintegration we know, N = N0eλt
If half life is designated by T, then, when t = T. N = N0/2
Then, N0/2 = N0eλt
or, eλt = 2
or, λt = loge2
then, T = loge2 / λ = (2.303 x loge2) / λ
= 0.6933/λ
That means, the half-life of a radioactive element is inversely proportional to the radioactive constant or decay constant of that element.
In order to disintegrate 1 g of uranium to 0.5 g of uranium, it will take 4500 million years. It will take another 4500 million years to disintegrate thus 0.5 g uranium to 0.25 g. So, the half-life of uranium is 4500 million years.
The half-life of isotopes from some sample elements:
- oxygen 16 – infinite
- carbon 14 – 5,730 years
- cobalt 60 – 5.27 years
- silver 94 – .42 seconds
Mathematical Example
What time will it take to decay 60% of a piece of radon? The half-life of radon is 3.82 days.
We know,
Half life, T = 0.6933/λ
or, 3.82 = 0.6933/λ
so, λ = 0.6933/3.82 = 0.1814 day.
If 60% of radon decays, then 40% of it remains i.e., of the 100% an initial number of atoms, 40% remains after decaying 60%.
so, N/N0 = 40/100.
Here, N0 = initial number of atoms and
N = rest number of atoms.
We know, N = N0eλt
then, N/N0 = eλt
or, 40/100 = e0.1814t or, 2/5 = e0.1814t
or, ln (2/5) = – 0.1814t
then, t = [ln 2/5] / – 0.1814t = 5.05
Ans. 5.05 days