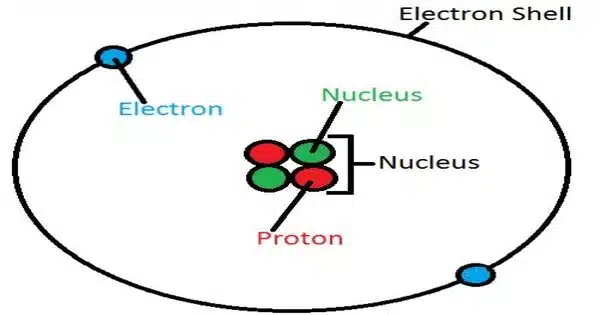
An atom of the chemical element helium is referred to as a helium atom. Helium is made up of two electrons held together by the electromagnetic force to a nucleus containing two protons and either one or two neutrons, depending on the isotope.
The atom of the element helium is known as a helium atom. It is one of the most basic and stable atoms, with two protons, two neutrons, and two electrons. Helium has an atomic number of 2, indicating that it has two protons in its nucleus. A helium atom’s two electrons occupy the first energy level in the electron cloud, with one electron in the 1s orbital and the other in the 1s* (1s-excited) orbital. The 1s orbital has the lowest energy and is closest to the nucleus.
In contrast to hydrogen, no closed-form solution to the Schrödinger equation for the helium atom has been discovered. However, approximations such as the Hartree-Fock method can be used to estimate the atom’s ground state energy and wavefunction.
Properties
- Atomic number: Helium has an atomic number of 2, which means it has two protons in its nucleus.
- Atomic mass: The atomic mass of helium is approximately 4 atomic mass units (AMU). This is the combined mass of two protons, two neutrons, and two electrons.
- Electron configuration: The electron configuration of helium is 1s2, indicating that it has two electrons occupying its 1s orbital.
- Electron shell structure: Helium has only one electron shell, as both of its electrons occupy the 1s orbital. This shell can hold a maximum of 2 electrons.
- Electronegativity: Helium is a noble gas and has a very low electronegativity. It does not readily attract or share electrons with other atoms.
- Stability: Helium is highly stable because its outermost shell is completely filled with electrons. This stability is why helium does not readily participate in chemical reactions.
- Density: The density of helium gas at standard temperature and pressure (STP) is about 0.1785 grams per liter (g/L). It is lighter than air and can cause balloons to float.
Helium is a noble gas that has a low reactivity. This is due to the fact that its outer electron shell is completely filled with two electrons, which makes it stable and unlikely to form chemical bonds with other atoms. It has the electron configuration 1s2, which indicates how the electrons are arranged in its energy levels.
Helium is lighter than air and has a variety of useful applications. Because of its extremely low boiling point, it is commonly used as a cryogenic coolant. It is also used in a variety of scientific and industrial processes, such as helium-neon lasers, deep-sea diving, and as an arc welding shielding gas.