Genetic engineering, also known as genetic modification, involves making precise changes to an organism’s genetic code. There are various methods for doing this, including CRISPR-Cas9, TALENs, and ZFNs. These methods use enzymes to cut DNA at specific locations, allowing for the insertion, deletion, or replacement of genetic material. The specific technique used will depend on the organism being modified and the desired outcome.
It is important to note that genetic engineering is a complex field and should only be attempted by trained professionals in a controlled laboratory setting. Additionally, any changes made to an organism’s genes can have unintended consequences and must be carefully evaluated before being implemented. CRISPR, the Nobel Prize-winning gene editing technology, is poised to have a profound impact on the fields of microbiology and medicine yet again.
A team led by CRISPR pioneer Jennifer Doudna and her longtime collaborator Jill Banfield has developed a clever tool to edit the genomes of bacteria-infecting viruses called bacteriophages using a rare form of CRISPR. The ability to easily engineer custom-designed phages – which has long eluded the research community – could help researchers control microbiomes without antibiotics or harsh chemicals, and treat dangerous drug-resistant infections. A paper describing the work was recently published in Nature Microbiology.
“Bacteriophages are some of the most abundant and diverse biological entities on Earth. Unlike prior approaches, this editing strategy works against the tremendous genetic diversity of bacteriophages,” said first author Benjamin Adler, a postdoctoral fellow in Doudna’s lab. “There are so many exciting directions here – discovery is literally at our fingertips!”
Phages have many ways to evade defenses, ranging from anti-CRISPRs to just being good at repairing their own DNA. So, in a sense, the adaptations encoded in phage genomes that make them so good at manipulating microbes are the exact same reason why it has been so difficult to develop a general-purpose tool for editing their genomes.
Benjamin Adler
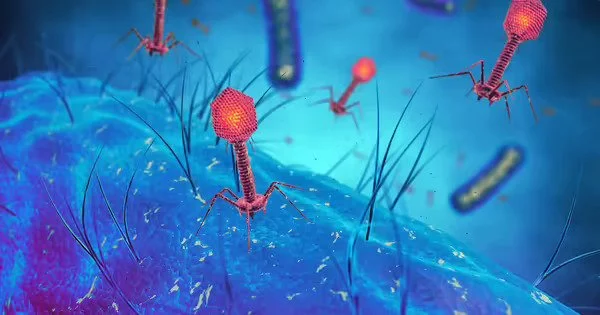
Bacteriophages, also known as phages, use a syringe-like apparatus to insert their genetic material into bacterial cells, then hijack the protein-building machinery of their hosts to reproduce themselves, usually killing the bacteria in the process. (They are harmless to other organisms, including humans, despite the fact that electron microscopy images show them to resemble sinister alien spaceships.)
CRISPR-Cas is an immune defense mechanism used by many bacteria and archaea against phages. A CRISPR-Cas system consists of short snippets of RNA that are complementary to sequences in phage genes, allowing the microbe to recognize when invasive genetic material has been inserted, and scissor-like enzymes that neutralize the phage genes by cutting them into harmless pieces after being guided into place by the RNA.
Over millennia, the constant evolutionary battle between phage offense and bacterial defense forced phages to specialize. Because there are many microbes, there are also many phages, each with its own set of adaptations. This astounding diversity has made phage editing difficult, including making them resistant to many forms of CRISPR, which is why the most commonly used system – CRISPR-Cas9 – doesn’t work for this application.
“Phages have many ways to evade defenses, ranging from anti-CRISPRs to just being good at repairing their own DNA,” said Adler. “So, in a sense, the adaptations encoded in phage genomes that make them so good at manipulating microbes are the exact same reason why it has been so difficult to develop a general-purpose tool for editing their genomes.”
Adler explained that the phage-fighting ability of CRISPR-Cas13 was unexpected given how few microbes use it. The scientists were taken aback because the phages it defeated all infected with double-stranded DNA, whereas the CRISPR-Cas13 system only targets and chops single-stranded viral RNA. Some phages, like other viruses, have DNA-based genomes, while others have RNA-based genomes. All known viruses, however, use RNA to express their genes. The CRISPR-Cas13 system effectively neutralized nine different DNA phages that all infect E. coli strains but have almost no genome similarity.
According to co-author and phage expert Vivek Mutalik, a staff scientist in Berkeley Lab’s Biosciences Area, these findings indicate that the CRISPR system can defend against diverse DNA-based phages by targeting their RNA after it has been converted from DNA by the bacteria’s own enzymes prior to protein translation.
Next, the team demonstrated that the system can be used to edit phage genomes rather than just chop them up defensively.
First, they created DNA segments with the desired phage sequence flanked by native phage sequences and inserted them into the phage’s target bacteria. When the phages infected the DNA-laden microbes, a small proportion of the phages reproducing within the microbes took up the altered DNA and incorporated it into their genomes in place of the original sequence. This is a long-established DNA editing technique known as homologous recombination. The long-standing issue in phage research is that, while the actual phage genome editing works perfectly, isolating and replicating the phages with the edited sequence from the larger pool of normal phages is extremely difficult.
This is where CRISPR-Cas13 comes into play. In the second step, the researchers modified another strain of host microbe to include a CRISPR-Cas13 system that detects and defends against the normal phage genome sequence. When the phages created in step one were exposed to the second-round hosts, the CRISPR defense system defeated the phages with the original sequence, but the small number of edited phages were able to evade it. They were able to survive and reproduce.
Experiments with three unrelated E. coli phages yielded an astounding success rate: more than 99% of the phages produced in the two-step processes had the edits, which ranged from massive multi-gene deletions to precise replacements of a single amino acid.
“This work on phage engineering is, in my opinion, one of the top milestones in phage biology,” Mutalik said. “Because phages influence microbial ecology, evolution, population dynamics, and virulence, seamless engineering of bacteria and their phages not only has profound implications for fundamental science, but it also has the potential to make a significant difference in all aspects of the bioeconomy. This phage engineering capability will have an impact on everything from biomanufacturing to agriculture to food production, in addition to human health.”