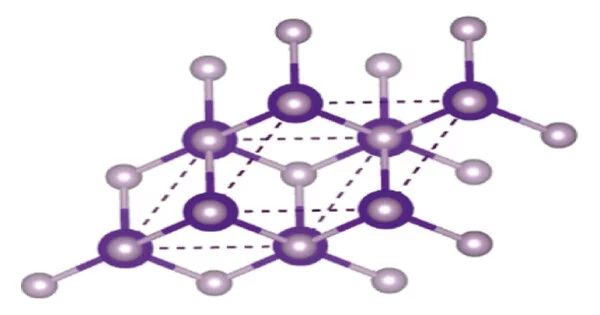
Indium Phosphide (InP) is a binary compound made up of indium and phosphorus atoms, with the chemical formula InP. It has a face-centered cubic (“zincblende”) crystal structure, identical to that of GaAs and most of the III-V semiconductors. It is a semiconductor material that has a wide range of applications in various fields, including optoelectronics, electronics, and solar cells.
Because of its superior electron velocity over the more common semiconductors silicon and gallium arsenide, it is used in high-power and high-frequency electronics. It also has a direct bandgap, which makes it suitable for optoelectronic devices such as laser diodes. When metal phosphides come into contact with water or stomach acid, they hydrolyze to phosphine.
Properties
- Chemical formula: InP
- Molar mass: 145.792 g/mol
- Appearance: black cubic crystals
- Density: 4.81 g/cm3, solid
- Melting point: 1,062 °C (1,944 °F; 1,335 K)
- Solubility: slightly soluble in acids
- Band gap: 1.344 eV (300 K; direct)
- Electron mobility: 5400 cm2/(V·s) (300 K)
- Thermal conductivity: 0.68 W/(cm·K) (300 K)
- Crystal structure: Zinc blende
Structure
InP has a zincblende crystal structure and a direct bandgap, which makes it an ideal material for the fabrication of optoelectronic devices such as photodetectors, laser diodes, and solar cells. Its bandgap energy is about 1.34 eV at room temperature, which is in the range of the visible spectrum.
Manufacturing
Indium phosphide can be prepared from the reaction of white phosphorus and indium iodide at 400 °C., also by direct combination of the purified elements at high temperature and pressure, or by thermal decomposition of a mixture of a trialkyl indium compound and phosphine.
InP is a relatively expensive material compared to other semiconductors, such as silicon and gallium arsenide, which limits its use in some applications. However, its unique properties make it a material of choice for certain specialized applications.
The reaction of white phosphorus and indium iodide results in the production of indium phosphide. Indium iodide is an orange-colored crystalline solid, whereas white phosphorus is a waxy and translucent solid that turns yellow when exposed to light. The following processes are used to produce indium phosphide:
- Both elements are set to 400 degrees Celsius.
- At high pressure and temperature, purified elements are combined.
- When a trialkyl indium compound and phosphine are thermally combined.
Applications
The application fields of InP splits up into three main areas. It is used as the basis for optoelectronic components, high-speed electronics, and photovoltaics. It is also used in high-frequency electronics applications, such as in high-speed transistors and microwave devices. Due to its high electron mobility and saturation velocity, InP is capable of operating at very high frequencies, which makes it an attractive material for use in high-speed digital and analog circuits.