Insulators in Solid Materials
A non-conductor or insulator has completely occupied lower band separated from the conduction band by a wide energy gap as schematically shown in Figure. At sufficiently high temperature the excitation of electrons from the valence band to the conduction band is negligible for insulator.
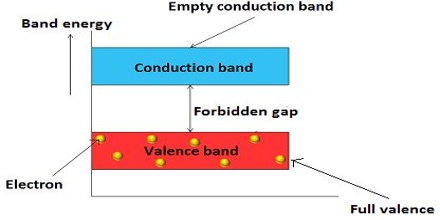
Fig: energy bands in an insulator; note large forbidden band
Materials with very low electrical conductivities are known as insulator. The electrical conductivity of semiconductors ranges from about 102 to 10-9 ohm-1 cm-1 at 25°C, while the maximum conductivity of a good conductor is about 10-4 ohm-1 cm-1 at 25°C. Substances having conductivity of the order of about 102 ohm-1 cm-1 at 25°C are known as insulators.
In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, having extremely high resistance to the flow of charge through them