Liquid-Liquid Equilibria in Partially-Miscible Systems: Triethylamine-water
There are pairs of substances which are liquids at ordinary temperatures or whose melting points are low or are known to have limited mutual solubility within certain temperature limits. They are said to be partly miscible in the temperature limits and their solubility curves form an interesting aspect of the application of the phase rule.
A simple and straightforward demonstration of the temperature dependence of partial miscibility is described. The Gibbs phase rule, temperature–composition phase diagrams, and the lever rule serve as the basis for an interpretation of the demonstration. These substances become completely miscible beyond the temperature limits. These limits are sometimes attained on raising the temperature or sometimes on lowering the temperature. In some systems, there are two limits, one higher and the other lower. In this section, an attempt will be made to discuss Triethylamine-water systems.
Triethylamine-water: This is a two-component system where it is found that the mutual solubility decreases on raising the temperature and increases in lowering the temperature. It is colorless to pale yellow transparent liquid, with a strong smell of ammonia, slightly fuming in the air. The behavior is just the reverse of the phenol-water system. Boiling point: 89.5 ℃, relative density (water = 1): 0.70, the relative density (Air = 1): 3.48, slightly soluble in water, soluble in alcohol, ether. The aqueous solution is alkaline, flammable.
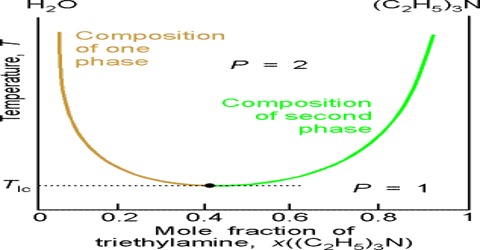
The solubility curve of water and triethylamine is shown schematically in Figure. At a temperature of 18°C and below the two components, water and triethylamine, are completely miscible in all proportions but above 18°C they are only partly miscible and two liquid layers L1 and L2 persist.
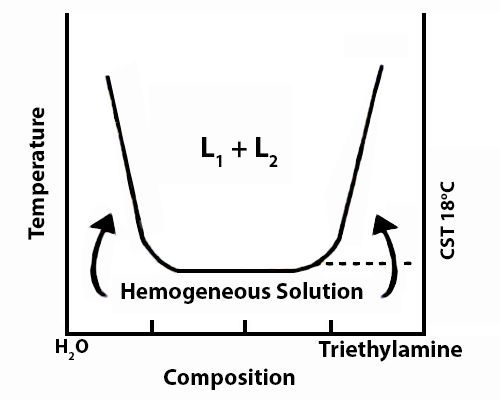
Fig: t-c diagram of water triethylamine
Critical solution temperature thus attained lowering the temperature is known as the lower consolute temperature in order to differentiate it from upper consolute temperature as in the case of phenol-water. Thus mutual solubility of two partly miscible liquids may be increased by raising or lowering the temperature depending on the system.
Critical solution temperature or consolute temperature may, therefore, be defined as the temperature above or below which two partly miscible liquids become completely miscible in all proportions. The water–triethylamine system is miscible in all mixing ratios at temperatures less than 18 °C, but separates into two immiscible phases for a wide range of bulk compositions as temperature increases through 19–24 °C. A procedure is described in which a range of bulk compositions simultaneously experiences an increase in temperature.
Triethylamine is mainly used in the production of quaternary ammonium compounds for textile auxiliaries and quaternary ammonium salts of dyes. Students are able to see differences in the temperature of phase separation, plus the evolving compositions and volumes of the separated phases.