Polymers are a class of materials in materials science that are made up of long chains of repeating molecular units called monomers. Molecular entanglements, which refer to the intertwining and interlocking of polymer chains, have a strong influence on the structure and properties of polymers. These entanglements are critical in determining the polymer’s mechanical, thermal, and viscoelastic properties.
The structure of semi-crystalline polymers is largely determined by the degree to which their molecular chains are entangled. Researchers developed a new model to predict the microscopic structure of the materials as well as their properties after conducting numerous experiments. Polymers are molecules with long chains. Semi-crystalline polymers are made up of a combination of solid and liquid elements. They’re commonly found in plastics and packaging materials.
The structure of semi-crystalline polymers is largely determined by the degree to which their molecular chains are entangled. This is demonstrated in a new study published in the scientific journal Proceedings of the National Academy of Sciences (PNAS) by researchers from Martin Luther University Halle-Wittenberg (MLU).
The researchers developed a new model to predict the microscopic structure of the materials as well as their properties after conducting numerous experiments. Polymers are molecules with long chains. Semi-crystalline polymers are made up of a combination of solid and liquid elements. They’re commonly found in plastics and packaging materials.
We believe our model can be applied to a wide range of polymers. This includes materials that are not currently widely used. The new findings could help to improve existing materials or replace them with more sustainable alternatives, either entirely or partially.
Thurn-Albrecht
When materials cool, they usually form a crystalline structure at the molecular level, which means that all of the particles are in a tightly ordered pattern. “A similar process occurs when semi-crystalline polymers form, except that not all regions crystallise,” explains MLU physicist Professor Thomas Thurn-Albrecht. Instead, there are amorphous regions, which have a disordered structure after cooling.
Entanglements that are intertwined with one another can be found here. On a nanoscale, ordered and disordered layers alternate. This unique structure also gives them their distinct properties: they are both flexible and elastic, as well as relatively robust. This makes them particularly suitable as packaging and structural materials.
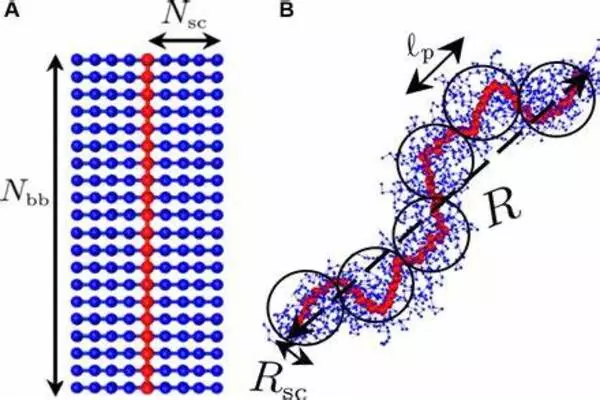
The properties of semi-crystalline polymers are heavily influenced by two factors: the thicknesses of the aforementioned layers and the degree to which the chains in the amorphous regions are entangled. The factors that influence crystal thickness, according to Thurn-Albrecht, are already well-known, but knowledge about amorphous layers is still rather limited. In collaboration with a group led by MLU professor Kay Saalwächter, his team investigated the process of crystal formation specifically for these layers.
When a polymer is formed, the polymerization process results in long chains of monomers that can interact with each other in several ways. One of the most important factors influencing the extent of molecular entanglements in a polymer is the degree of polymerization. The degree of polymerization refers to the number of monomer units in a single polymer chain. Longer polymer chains have a higher probability of entangling with neighboring chains, leading to a more entangled polymer structure.
The physicists discovered that the thickness of the amorphous layers is determined to a large extent by their entanglements based on measurements on a model polymer. In addition, the researchers created a simple model to describe this relationship.
“We believe our model can be applied to a wide range of polymers. This includes materials that are not currently widely used,” says Thurn-Albrecht. The new findings could help to improve existing materials or replace them with more sustainable alternatives, either entirely or partially.”