Nature of Adsorption
Commonsense suggests that if adsorption results from purely physical forces like van der Waals forces it will have a pattern or nature quite different from the nature of adsorption caused by chemical forces since physical forces and chemical forces are of different nature. Consequently, two limiting types of adsorption are recognized:
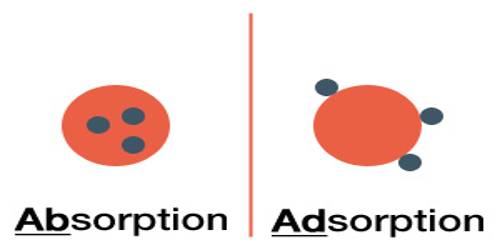
(a) Physical adsorption: When a particle is held on the solid surface by purely physical forces like van der Waals forces, and
(b) Chemical adsorption or Chemisorption: Chemisorption (also referred to as chemical adsorption) refers to a phenomenon in which there is a chemical interaction between adsorbent and adsorbate. In this case, the binding force between adsorbent and adsorbate is relatively strong and it can be considered that a true covalent chemical bond is formed. Energy changes in chemisorption are much higher than in adsorption. The term ‘activated adsorption’ is often used in place of chemisorption.
However, in many cases, there may be partly physical adsorption and partly chemisorption, both occurring simultaneously on the surface. These cases are complicated and no attempt will be made to explain their behavior.
When a gas molecule freely moving at random in all directions in the gas phase is adsorbed on a surface its translational freedom is lost or curtailed due to its adherent, on the surface and its random motion is lost. If the adherence is strong it completely loses its translational motion and its vibration and rotational energies, are also modified. If the adherence is not strong it can at least move around within restricted areas of the surface but such motion must be less than the motion in the gas phase. Consequently, such adsorption must be accompanied by a rise in temperature. i.e., adsorption shall be exothermic. The part or whole of the translational kinetic energy is conveyed to heat energy. On the other hand, if adsorption is caused by chemical effects, the overall energy change will be governed by the nature and magnitude of chemical forces involved, plus the energy released due to loss of kinetic energy. No prediction from purely kinetic consideration can be made. Indeed chemisorption is quite complex and most of the discussions to follow will be confined mainly to physical adsorption.