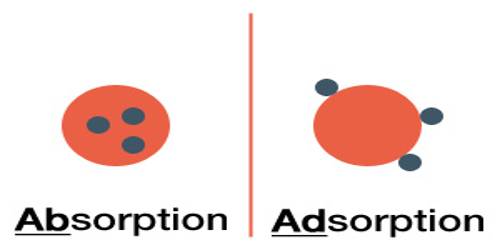
Adsorption on the surface of a material may be defined “as a process in which the concentration of a chemical species is greater on the surface than in the bulk resulting from inelastic collision suffered by molecules on the surface.” The species that is adsorbed is called adsorbate and the material of the surface on which adsorption takes place is called adsorbent. Adsorption strictly refers to an accumulation of adsorbate on the surface only due to the residual field of force. An adsorbent attracts an adsorbate or adsorbed phase by van der Waals type of relatively weak forces. Two examples of adsorption are given below:
(a) Adsorption of ethanoic acid by charcoal: If powdered charcoal is added to a solution of ethanoic acid and the solution allowed to stand for some time, it is found that the concentration of the acid in the solution has decreased. This is because some of the acids are adsorbed by the charcoal on its surface.
(b) Adsorption of a gas by a solid: In an enclosed vessel containing SO2 or NH3 gas some powdered charcoal is added and the pressure measured before and after addition of the solid. It is found that the pressure of the gas has decreased. This is attributed to adsorption of the gas by the charcoal.