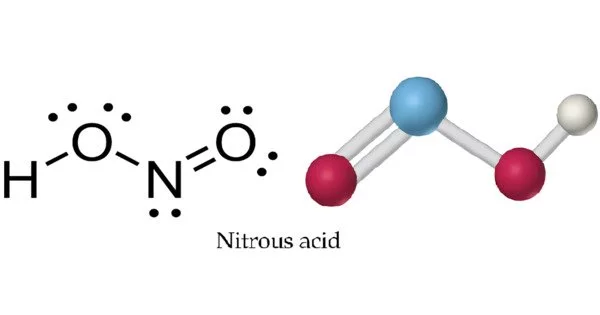
Nitrous acid (molecular formula HNO2) is a weak and monoprotic acid that is only known in solution, gas phase, and as nitrite (NO2) salts. It is made up of atoms of hydrogen (H), nitrogen (N), and oxygen (O). It is well-known for its participation in a variety of chemical reactions as well as its significance in chemistry and biology. It is employed in the synthesis of diazonium salts from amines. The diazonium salts formed are used as reagents in azo coupling processes to produce azo colors.
In solution, nitrous acid occurs as a mixture of its tautomeric forms, which include the more stable nitrosyl-hydroxide (HNO2) and the less stable nitroxyl (HNO) and hyponitrous acid (H2N2O2). The precise distribution of these species is determined by parameters such as pH and temperature.
Properties
- Chemical formula: HNO2
- Molar mass: 47.013 g/mol
- Appearance: Pale blue solution
- Density: Approx. 1 g/ml
- Melting point: Only known in solution or as gas
- Acidity (pKa): 3.15
- Conjugate base: Nitrite
Preparation
Nitrous acid is usually generated by acidification of aqueous solutions of sodium nitrite with a mineral acid. The acidification is usually conducted at ice temperatures, and the HNO2 is consumed in situ. Free nitrous acid is unstable and decomposes rapidly.
Nitrous acid can also be produced by dissolving dinitrogen trioxide in water according to the equation
N2O3 + H2O → 2 HNO2
Chemical Properties
It is a weak acid, meaning it partially dissociates in water to release hydrogen ions (H+). It is also a reducing agent, capable of donating electrons in chemical reactions. Nitrous acid is unstable and decomposes readily, especially at elevated temperatures, into nitrogen oxides and water.
Biological Importance
Nitrous acid is also involved in biological activities. It is generated in the human body as a result of the metabolism of certain medicines and meals. Nitrous acid and its derivatives have been investigated for its possible role in nitrosative stress and their link to specific health disorders.
Safety
Nitrous acid is a very unstable and potentially harmful substance. It can spontaneously dissolve, generating harmful nitrogen oxides and, in some situations, causing explosive reactions. As a result, it should be handled with caution in a controlled laboratory setting.