Physical Properties of Carboxylic Acids:
(i) Physical state: The first nine members (C1 ≈ C9) are colorless, pungent smelling liquids. The higher members are colorless, odourless waxy solids. Carboxylic acids are acidic because of the hydrogen in the -COOH group. When the acids form salts, this is lost and replaced by a metal.
(ii) Solubility: The acids are polar. The – COOH group forms H-bond with H2O. The lower members of the aliphatic carbon lie acid family (upto C4) are highly soluble in water. The solubility decreases with the increase in the size of the alkyl group. Carboxylic acids having one to four carbon atoms are completely miscible with water. Solubility decreases with molar mass. All carboxylic acids are soluble in alcohol, ether, benzene etc.

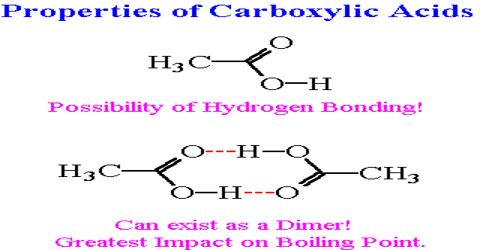
(iii) Boiling point: The carboxylic acid molecules can form dimmer by intra-molecular H-bond. The boiling points of the carboxylic acids increases with molar mass, i.e. an acid containing larger number of C atoms has higher boiling point. Carboxylic acids exhibit strong hydrogen bonding between molecules. They therefore have high boiling points compared to other substances of comparable molar mass.