The electromagnetic theory, however, failed to account for the phenomenon of photo electric effect. In 1900, Planck had suggested that energy was emitted and absorbed, not continuously but in multiples of discrete pockets of energy called Quantum which could not be subdivided into smaller parts. In 1905, Einstein extended this idea and suggested that light waves consist of small pockets of energy called photons. The energy associated with each photon is E = hν, where h is Planck’s constant (h = 6.626 × 10-34 Js) and ν is the frequency of the electromagnetic radiation.
It is now established that photon seems to have a dual character. It behaves as particles in the region of higher energy and as waves in the region of lower energy (Figure).
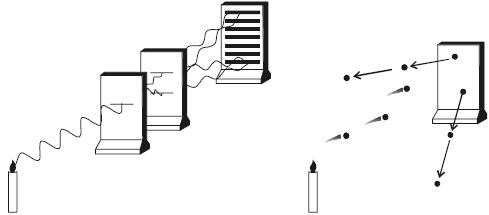
Fig: Wave and Quantum nature