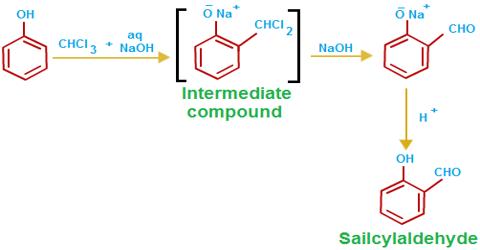
Chloroform is a colorless, volatile, liquid derivative of trichloromethane with an ether-like odor. Formerly used as an inhaled anesthetic during the surgery, the primary use of chloroform today is in industry, where it is used as a solvent and in the production of the refrigerant freon.
Reactions of Chloroform:
(i) Oxidation: It undergoes oxidation in the presence of light to give a highly poisonous substance called phosgene.
2CHCl3 + O2 → sunlight→ 2COCl2 + 2HCl.
(ii) Substitution: Chloroform reacts with nitric acid to form chloropicrin or nitro-chloroform. It is one of the components of the tear shell.
Cl3C – H + HNO3 → Cl3C – NO2 + H2O.
(iii) Reduction: When chloroform is refluxed in the presence of zinc dust and water, chloroform is reduced to form methane.
Zn + 2H2O → Zn(OH)2 + 2[H]
CHCl3 + 6(H) → CH4 + 3HCl
(iv) Acidic Property: CHCl3 forms sodium/potassium formate by boiling with alcoholic NaOH/KOH solution.
CHCl3 + 4NaOH → H-COONa + 3NaCl + 2H2O
(v) Carbonyl Amine Reaction: When chloroform is heated (60°-70°C) with aniline and caustic potash, phenyl isocyanide with extremely intolerable odor is obtained. This reaction serves as a test for chloroform or aniline identification.
(vi) Potassium hydroxide Reaction: When chloroform reacts with aq. KOH, the chlorines on the carbon atom are successively replaced by -OH groups from KOH via. nucleophilic reaction (SN2). 1 mole of Chloroform will react with 4 moles of KOH to produce 1 mole of HCOOK (potassium formate), 3 moles of KCl, and 2 moles of H2O.
1 CHCl3 + 4 KOH → HCOOK + 3 KCl + 2 H2O.
This is if you allow the reaction to go to completion. If the reaction conditions are not severe enough, you can isolate some of the intermediates as well.
Properties of Chloropicrin:
- It is an oily substance.
- It is used in a tear-gas shell as it is a tear-producer.
Precaution
Acute chloroform toxicity results in impaired liver function, cardiac arrhythmia, nausea, and central nervous system dysfunction. As a byproduct of water chlorination, chloroform may be present in small amounts in chlorinated water.