Rutherford’s Alpha Particle Experiment
Throughout the nineteenth century, scientists had the idea that each atom was composed of positive charges and these charges were distributed all over the atom. Within this positively charged matter, negatively charged electrons were randomly distributed. Each atom contained the same number of positive and negative charges.
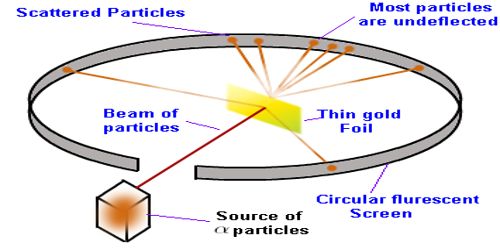
In 1909, scientists Geiger and Marsden, at the suggestion of Ernest Rutherford, performed a deflection experiment of alpha-particles; having an energy of 7.68 MeV emitted from the radioactive polonium, on a 6 x l0-7 m thick gold foil [Figure 1]. This experiment is known as the famous Rutherford’s alpha deflection experiment. In this experiment, they noticed that some alpha particles went straight through the foil and some particles were scattered making small angles and some went making the angle more than 90°. Some particles even scattered in the backward direction making 180° angle.
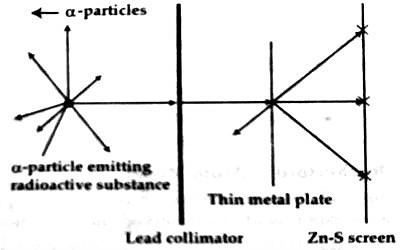
Fig: (1)
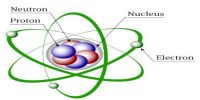
From this experiment, Lord Rutherford first proposed that scattering of α-particles through large angles is only possible if all the positive charges and the mass of the atom are concentrated at the center covering only a very small space around it. Later on in 1913, complete agreement of Rutherford’s proposal was found with the experimental results of Geiger and Marsden. From this experiment, Rutherford reached the conclusion that total positive charges and mass of the atom were concentrated at the center of a very small space. Scientist Rutherford named it as the nucleus. So, the credit of discovery of nucleus goes to Rutherford Nucleus is the heart or source of energy of the atoms i.e. the matter.
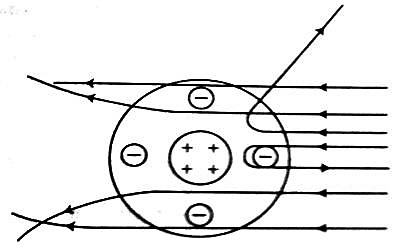
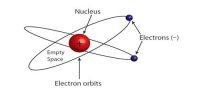
Fig: (2)
Explanation of the results of the experiment: According to Rutherford all the positive charges and the mass of the atom are concentrated at the center of the atom [Figure 2]. Electrons are distributed randomly around the nucleus. The probability of coming closer to the nucleus of the positively charged alpha particles is less while going through the gold foil. So, most of the alpha particle go straight through the empty space of the atom. Again, those alpha particles which tome very close to the nucleus will be repelled by the positive charge of the nucleus and will be deflected from their initial paths. Besides, those alpha particles which approach directly the nucleus will encounter intense electric field and according to the Coulomb’s inverse square law these particles will be repelled by maximum force and will be scattered in the backward direction making an angle of 180°.
It is to be mentioned that alpha particles are about 7000 times heavier than electrons and they strike the gold foil with tremendous speed. So it can safely be said that electrons inside the atoms of the gold foil being so light do not appreciably affect the motion of incident alpha particles and can never scatter the alpha particles to such a large angle of 180′ or to any other large angles.
By analyzing the deflection of the alpha particles and the results obtained, Rutherford put forward a model of an atom. This is called Rutherford’s atom model.