Amide bonds are essentially chemical compounds in proteins and peptides and have a long-established role in small-molecule therapists. Linking molecular components by amide bonds is one of the most important reactions in the science and chemical industries. Scientists have now introduced a new form of reaction for producing amide bonds in the journal Anwande Chemie. Called ASHA ligation, this reaction is rapid, efficient, occurs under mild aqueous conditions, and generally applies.
Despite their known significance, the amide bond synthesis depends almost entirely on variations of a single reaction: the dehydrative coupling of carboxylic acids and amines. Although phenomenally efficient in many applications, this method involves superstoichiometric amounts of costly reagents and prevents chemoselective ligation reactions.
Amid bonds is the bond between a carbonyl carbon (C=O) and an organic nitrogen atom. It is an amide bond that links individual amino acids together into proteins and binds monomers into polyamide plastics such as perlon and nylon. Many lives-saving drugs, such as taxol, lipitor, and penicillin, as well as agrochemicals, organic conjugates, natural substances, and other materials, contain amide bonds. In addition to the classical method of production-the interaction of an acid group (-COOH) with an amino group (-NH(2))-a number of different reactions have been produced for the formation of amide bonds. However, all of them are not necessarily available due to lack of chemoselectivity. They also require specialized coupling agents. New types of reactions are in demand.
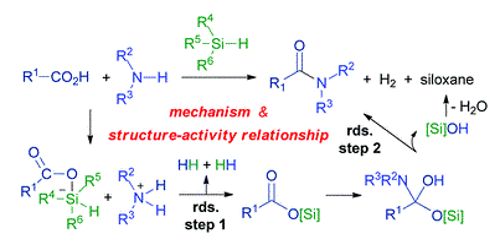
A team led by Rajavel Srinivasan at Tianjin University (China) was looking for an effective, sustainable approach that begins with easily accessible materials and works quickly. They preferred a reaction between acylsilans and hydroxylamines (a class of organic nitrogen compounds with an -N-O- linkage). Acylsilans are a family of organic silicon compounds with a general molecular formula of R(CO)-SiR. (3). Their reactivity is also somewhat different from that of the associated ketones. Although their chemistry is well known, it has seldom been used in the field of biomedical chemistry.
Generally, two fundamental techniques, i.e. thermodynamic and kinetically regulated strategy, are differentiated within the protease-catalyzed peptide bonding approaches. The thermodynamic approach reflects a direct reverse of catalytic proteolysis and finishes with a true balance. Since this balanced-controlled method is an endergonic operation, it is important to manipulate the reaction conditions in order to change the balance towards the peptide product in order to maximize the yield of the coupling reaction.
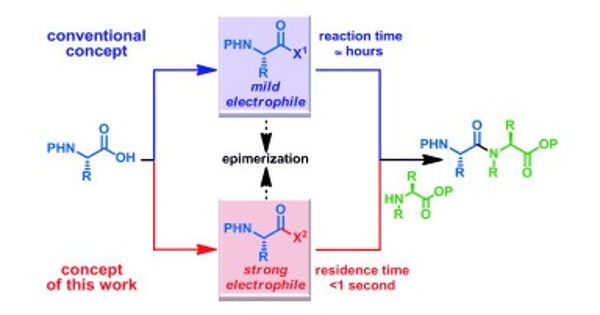
Centered on their first letters, the reaction of acylsilanes to hydroxylamines is termed ASHA ligation. The driving force tends to be the intramolecular movement of the silyl group from the carbon atom to the oxygen atom (Brook re-arrangement) in which a tight Si-O bond is formed.
Researchers have successfully examined a wide variety of various molecular building blocks, including medicinal agents, peptides, natural products, and other biologically active compounds. The reaction is chemoselective and works under moderate conditions in an aqueous environment. In most cases, it provides high yields and is tolerant of most functional groups. New variants of ASHA ligation are in progress for peptide synthesis, as they did not perform well.
However, the versatility and reliability of the ASHA ligation should open pathways to new methods in medicinal chemistry and chemical biology, such as fragment-based drug production, in which active compounds are made up successively from small fragments.