Secondary Standard Electrodes
An electrode with an invariant potential. In electrochemical methods, where it is essential to monitor, determine, or organize the potential of another electrode (denoted indicator, test, or working electrode), it is required to use a reference electrode, which maintains a potential that remains almost unchanged during the course of electrochemical measurement.
The hydrogen electrode is not a convenient reference electrode to use in measurement for the following reasons:
(i) One has to prepare a Standard Hydrogen Electrode (SHE) every time it is needed; the procedure is cumbersome.
(ii) Maintaining a stream of hydrogen at 1 atm takes careful arrangement,
(iii) Preparation of a solution of an acid in which the concentration of H+ ion is exactly 1.0 mol L-1 is time-consuming.
(iv) It is not easy to prepare pure hydrogen.
(v) Hydrogen has to be handled very carefully as it is inflammable.
One such electrode, the usual hydrogen electrode, has been preferred as a reference standard, relative to which potentials of other electrodes and those of oxidation-reduction couples are often expressed. The hydrogen electrode itself is used only in basic studies and some nonaqueous solutions. The hydrogen electrode, however, remains essential for providing a reference standard.
In order to avoid these difficulties other electrodes that can be prepared easily and whose electrode potentials are constant under certain conditions have been devised. Such electrodes are known as secondary standard electrodes. Two such electrodes arc:
- Calomel Electrodes
- Silver Electrodes
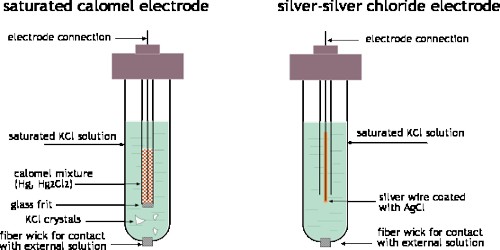
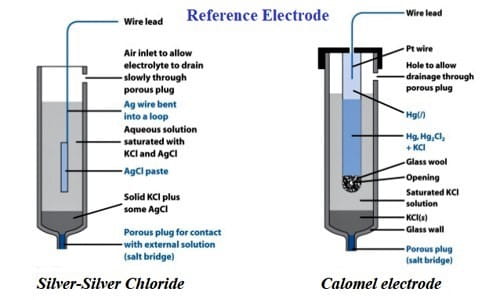
Calomel electrode: This reference electrode consists of mercury and mercury-chloride molecules. This electrode can be comparatively easier to make and sustain compared to the SHE.
Calomel electrodes represented as- Pt│Hg.Hg2 Cl2(s).KCl (salt)(aq)
The electrode reaction is- Hg2 Cl2(s) + 2e– = 2Hg (l) + 2Cl– (aq)
The potential of the saturated calomel electrode is 0.244 V.
This saturated solution allows for the exchange of chlorine ions to take place. All this is generally placed inside a tube that has a porous salt bridge to allow the electrons to flow back through and entire the circuit.
Silver Electrodes: An electrode of this variety precipitates a salt in the solution that participates in the electrode reaction. This electrode is prepared by dipping a silver rod into a saturated solution of potassium chloride over a paste of AgCl and is represented as-
Ag│AgCl(s), Cl– (salt)(aq)
The electrode reaction is- AgCl(s) + e– ↔ Ag (s) = Cl– (aq)
A Silver-Silver Chloride electrode is made by taking a wire of solid silver and coding it in AgCl. This is an extensively used reference electrode because it is economical and not as toxic as the Calomel electrode that contains mercury.