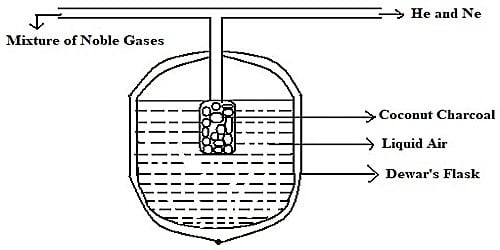
The individual noble gases can be separated by Dewar Charcoal adsorption method.
The principle involved in Dewar’s method:
- Activated coconut charcoal adsorbs all the Noble gases excluding Helium.
- The adsorption depends on temperature. Lower the atomic weight of the Noble gas, the lower is the temperature needed to absorb it.
The adsorption capacity power of difference noble gases on charcoal depends on two things:
- At low temperature, the adsorption capacity of these gases increases with the increase in the atomic weight of noble gases.
- Adsorption capacity also depends on temperature and it is inversely proportional to the temperature.
The mixture of noble gases obtained by the above method is separated into individual constituents by the use of coconut charcoal which adsorbs different gases at different temperatures.
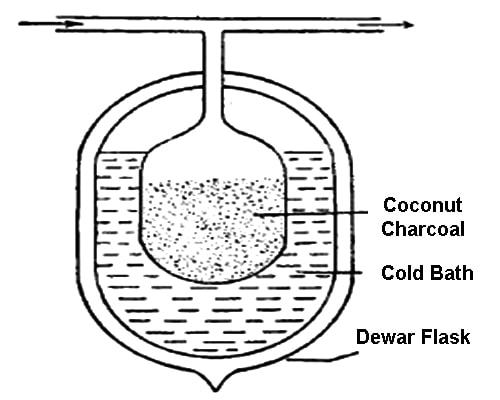
Fig: Separation of noble gases (Dewar’s method)
Explanation:
The mixture of noble gases is passed into a double-walled bulb containing coconut charcoal and placed in a low-temperature bath at 173K. It is allowed to remain in contact with the charcoal for about half an hour. At 173K, only argon, krypton, and xenon are adsorbed by the charcoal while helium and neon remain unadsorbed. These are pumped out and collected.
The mixture of helium and neon is kept in contact with coconut charcoal at 93K which completely adsorbs neon leaving free helium.
The charcoal at 173K containing argon, krypton, and xenon is placed in contact with another charcoal at the temperature of the liquid air when argon diffuses into the other charcoal. Here noble gases are separated by fractional distillation of liquid air using the difference in the boiling points of these using Claude’s apparatus.
The temperature of the first charcoal (temp.173K) still containing krypton and xenon is raised to 183K when Krypton is set free while xenon remains adsorbed in the charcoal. When it is heated, xenon is recovered.
Procedure: Separation of noble gases is carried out in a flask designed by Dewar called Dewar’s flask. It is a double walled flask containing activated coconut charcoal in the central point. A tube is arranged above the flask, to introduce the gaseous assortment and to remove the unadsorbed gases. The procedure involves the following steps.
- The mixture of noble gases is brought in contact with charcoal (Charcoal -1) kept in a Dewar’s flask maintained at a temperature of 173K. At 173 K, Ar, Kr and Xe gases are absorbed by the coconut charcoal while He and Ne remain unadsorbed. It is allowed to remain for one hour, when argon, krypton, and xenon gases get adsorbed while helium and neon remain unadsorbed and are pumped out of the bulb.
- The mixture of helium and neon is introduced into another bulb containing coconut charcoal (Charcoal – 2) maintained at a temperature of 93K. The mixture of He and Ne is introduced into another Dewar’s flask and 93 K temperature is maintained. At 93 K, only Ne gets adsorbed leaving behind He. Only neon gets adsorbed leaving behind helium which is pumped out. This charcoal is warmed to recover neon.
- The first charcoal with Argon, Krypton, and Xenon adsorbed on it is brought in contact with another charcoal (Charcoal – 3) cooled to liquid air temperature (11K). The charcoal with Ar, Kr, Xe is cooled to 77K. Argon separates out. Argon being a lighter gas diffuses into this charcoal (at 77K)and is recovered by warming separately.
- The temperature of the charcoal raised to 183 K. At this temperature, Kr separates out. Xenon which remains on the first charcoal is released by warming. At this temperature, krypton cannot remain in the adsorbed state and it is collected. Xenon which remains on the first charcoal is released by warming and is collected separately. All these changes are shown schematically.