The significance of Universal Gas Constant (R)
The gas constant is, by convention, symbolized R. The gas constant is also well-known as the molar, worldwide, or ideal gas constant, denoted by the symbol ‘R’. It is a physical constant that appears in an equation defining the behavior of a gas under theoretically ideal conditions. It is corresponding to the Boltzmann constant but articulated in units of energy per temperature augmentation per mole, i.e. the pressure-volume product, rather than energy per temperature increase per constituent part.
The gas constant, also known as the universal gas constant, is a physical constant that appears in an equation defining the behavior of a gas under theoretically ideal conditions.
When a mole of gas is considered it follows from the equation that: PV = RT
or, R = (PV)/T …. ….. …. … (1)
R is called the universal gas constant or molar gas constant. It follows from equation (1) that for one mole of any gas if the pressure is P1, the volume is V1 at temperature T1 then at temperature T2 the pressure P2 and volume V2 would be such that;
(P1V1)/T1 = (P2V2)/T2
This relation is true for a known quantity of any gas.
The numerical value of R may be calculated by determining the volume occupied by 1 mole of a gas at a given pressure and a given temperature. The dimensions of R may be easily deduced from equation (1).
At a temperature much superior to the decisive temperature and at low pressures, however, the ideal gas law is a very good model for gas behavior. The pressure, volume, amount of material, and temperature of a gas are associated according to the following equation, known as the general gas equation:
PV = NRT
where P represents the pressure in pascals, V represents the volume in meters cubed, N represents the amount of essence in moles, and T represents the absolute temperature in kelvins.
In terms of the pressure, volume, amount of substance, and temperature, the gas constant is equal to:
R = (PV) / (NT)
P is pressure, V is volume, n is the number of moles, and T is temperature.
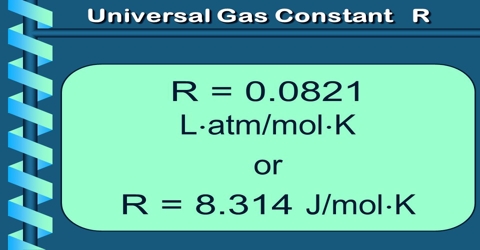
The gas constant has been found, by experimentation, to have a value of more or less 8.3145 joules per kelvin per mole (J·K-1·mol-1).
The ideal gas constant is a worldwide constant that we use to enumerate the connection between the properties of a gas. The constant R that we obviously use relates to pressure in atmospheres, volume in liters, and temperature in Kelvin. When dealing with gases at low temperature and at high pressure, modification has to be made in order to analyze the properties of a gas in manufacturing and technical applications.
Example: A 1.00 L flask was filled up with gas at a pressure of 751 mm Hg temperature 26°C. What volume would this gas occupy at STP?
Solution: The information given is as follows;
P1 = 751 mm Hg; P2 = 760 mm Hg
V1 = 1.00 L; V2 = Unknown
T1 = 299 K; T2 = 273 K
Substituting these data in equation (1) and solving for V2 we get,
V2 = (P1V1)/T1 . (T2/P2) = [(751*1.00)/299]*(273/299) = 0.90 L