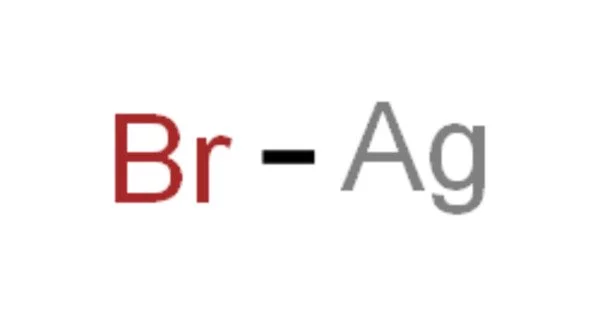Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt that is well known for its unusual sensitivity to light (along with other silver halides). It is an AgBr compound that is extremely light sensitive and is widely used in photographic materials. This property has allowed silver halides to become the foundation of modern photographic materials. A cubical silver bromide crystal is made up of an orderly array of silver and bromine.
AgBr is a light sensitive compound that is widely used in photography. It is widely used in photographic films and is thought by some to have been used in the creation of the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite.
Properties
Silver bromide is found as a pale-yellow odorless solid. It has a density of 6.473 g/mL, melting point of 432 °C and boiling point of 1,502 °C. It is insoluble in water.
- Chemical formula: AgBr
- Molar mass: 187.77 g/mol
- Appearance: Pale yellow solid photosensitive
- Density: 6.473 g/cm3, solid
- Melting point: 432 °C (810 °F; 705 K)
- Boiling point: 1,502 °C (2,736 °F; 1,775 K) (decomposes)
- Solubility in water: 0.140 mg/L (20 °C)
- Solubility product (Ksp): 5.4 × 10 −13
- Solubility: insoluble in alcohol, most acids sparingly soluble in ammonia
The photosensitive property of silver bromide distinguishes it (absorbs energy from light). When exposed to light, it reacts by turning grey or black. As a result, it must be kept in the dark. It readily reacts with liquid ammonia to form a variety of amine complexes. Under normal conditions, it is a stable compound, but when heated to high temperatures, it decomposes, releasing toxic bromine fumes.

Preparation
Industrially, silver bromide is produced by reacting aqueous solutions of silver nitrate and potassium bromide. At the end of the reaction, the water-insoluble silver bromide product precipitates out, while the potassium nitrate by-product remains in solution.
Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide:
AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq)
Although less convenient, the compound can also be prepared directly from its elements.
Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to produce small, uniform crystals (typically < 1 μm in diameter and containing ~1012 Ag atoms) called grains.
Occurrence
Silver bromide occurs naturally as the mineral bromargyrite in considerable amounts. However, it is typically obtained in large amounts through chemical production.
Uses
Silver bromide’s main applications are in photography due to its light sensitivity. It is used in the production of photographic films and plates. It’s also used in infrared technology, light-sensitive eyeglasses, and semiconductors. It has antiseptic properties and is used as a topical disinfectant and astringent, similar to other silver halides.
Health effects
Silver bromide is not a particularly dangerous substance. It, like many other silver compounds, can cause irritation and greyish discoloration when it comes into contact with the skin or eyes. Sneezing, coughing, and difficulty breathing can occur as a result of inhalation. Swallowing large amounts can discolor tissue and harm the central nervous system and kidneys.