A new study sheds light on the molecular mechanisms underlying the body’s natural defenses against the development of skin cancer. The protein CSDE1 orchestrates a complex chain of events that allows skin cells to age. Senescent cells act as a cancer-fighting barrier, preventing tumor formation. The findings are surprising because CSDE1 has previously been linked to cancer formation. The findings provide new insights into the cellular behavior of skin cancer, paving the way for potential new therapeutic targets to treat the disease.
A study published in Cell Reports provides important insights into the molecular mechanisms that underpin the body’s natural defenses against skin cancer development. The findings provide new insights into the cellular behavior of skin cancer, paving the way for potential new therapeutic targets to treat the disease.
“We discovered that the protein CSDE1 coordinates a complex chain of events that enable senescence in skin cells, significantly slowing their function without causing death,” says Rosario Avolio, the study’s first author and postdoctoral researcher at the CRG at the time of submission. “The resulting cells act as a cancer-fighting firewall, suppressing tumor formation.”
We discovered that the protein CSDE1 coordinates a complex chain of events that enable senescence in skin cells, significantly slowing their function without causing death. The resulting cells act as a cancer-fighting firewall, suppressing tumor formation.
Rosario Avolio
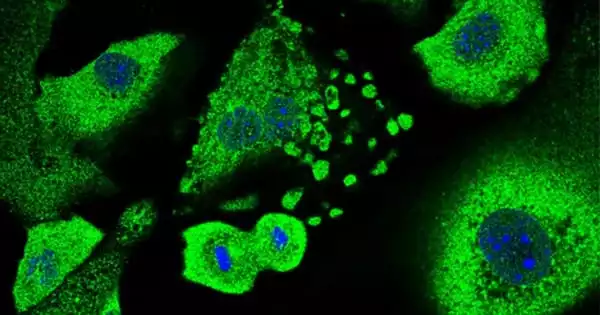
The study was carried out by researchers at the Centre for Genomic Regulation (CRG) led by Fátima Gebauer. Keratinocytes, the most abundant type of skin cell in the epidermis, were collected from mice. Keratinocytes can cause a variety of skin cancers, including basal and squamous cell carcinomas, two of the most common types of human cancer.
The researchers experimentally introduced genes that promote cancer formation, causing the cells to enter a dormant state. They discovered that when CSDE1 levels were reduced, cells were unable to undergo senescence and became immortalized, a necessary step in the development of cancer.
Further research found that when CSDE1-depleted cells were implanted under the skin of mice, they began to form malignant tumors. This was striking to the authors because every treated mouse developed squamous cell carcinomas within 15 to 20 days, emphasizing the importance of CSDE1 in tumor suppression.
The researchers discovered that CSDE1 suppresses tumors via two distinct mechanisms. CSDE1 causes the cell to secrete a cocktail of cytokines and enzymes, causing it to enter a permanent growth arrest. CSDE1 also inhibits the synthesis of YBX1, a protein previously implicated in tumor growth and aggressiveness.
The study’s findings, according to the authors, are surprising because CSDE1 has previously been linked to driving the formation of cancers rather than suppressing them. Previous research led by Dr. Gebauer found that CSDE1 promotes the formation of metastases in melanoma, a less common but more aggressive type of skin cancer. Other research has linked CSDE1 to tumor proliferation in a variety of cancers.
“CSDE1 is the protein equivalent of ‘Dr. Jekyll and Mr. Hyde.’ It has an unpredictability in its dual nature depending on the type of cell and tissue in which it is found “Dr. Gebauer, acting co-coordinator of the CRG’s Gene Regulation, Stem Cells, and Cancer research program and senior author of the study, explains. “We don’t know why this protein causes cancer in some people while suppressing it in others. Investigating the underlying cause will have significant implications for the development of new, more personalized cancer treatments.”
CSDE1 is an RNA-binding protein, which is a type of protein that monitors RNA, often as soon as it is made, with the potential to significantly alter its function. One possible explanation for why CSDE1 behaves differently is that normal skin cells and tumors have slightly different variants of the protein that affect the larger molecular machinery in different ways.
The study is one of the few to investigate the role of RNA-binding proteins in the establishment of cell senescence, which is an important new frontier in cancer research. “It was long thought that RNA-binding proteins were universal molecules that cells used for general housekeeping and could not be targeted therapeutically. It is becoming increasingly clear that this is not the case, and that this emerging field is critical for understanding human disease “Dr. Gebauer comes to a close.