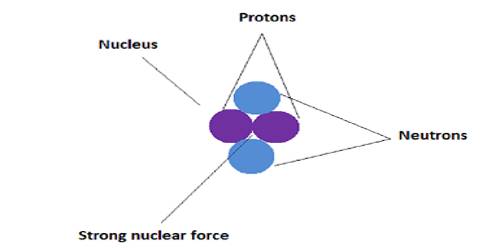
Strong Nuclear Force
A nucleus of an atom is composed of protons and neutrons. These are combined called nucleons. Since similar positively charged protons remain very close to each other, hence Coulomb’s repulsive force between them should be very strong and consequently, the nucleus should have broken. In reality, many nuclei are stable and the existing nuclear force does not allow the nucleus to break. The gravitational force that is active between the nucleons is so negligibly small that it cannot balance the Coulomb’s repulsive force. So, there must exist a strong nuclear force which holds the nucleus together. This force is called strong nuclear force. Scientists think that by the mutual exchange of a particle named meson inside the nucleus this force becomes active. This force is attractive and is not effective outside the nucleus. That means this force is effective within short range. This force is attractive and independent of charge.
Strong nuclear force is the force that binds nucleons in the nucleus together. Neutrons and proton are called nucleons. Protons have a positive charge, but neutrons do not have any charge. Now in the nucleus, all these particles are closely packed. Naturally, there would be a strong repulsive force between protons as they have similar charges. So this repulsive force should break the nucleus into pieces. Since it does not happen, so there must be a strong force which keeps the nucleus together in the nucleus. This strong force is called the strong nuclear force.