Cognitive impairment is very common in the aged population. As peripheral mitochondrial activity is connected with moderate cognitive impairment (MCI) in the older population, evidence shows that mitochondrial function in lymphocytes may be possible indicators in the progression of neurodegeneration. Mitochondrial dysfunction is a frequent hallmark of many hereditary illnesses that affect cognition and the brain. However, the precise involvement of these organelles in the development of such illnesses is unknown.
Increased oxidative damage has been linked to neurological diseases like Alzheimer’s (AD). Despite extensive research into the pathogenesis of Alzheimer’s disease, the potential association between mitochondrial malfunction and the disease remains largely unknown. Prof. Koji Fukui of Japan’s Shibaura Institute of Technology led a research team that confirmed that Alzheimer’s disease progression is connected to oxidative brain damage, which impairs cognitive function in AD transgenic mice in an age-dependent manner.
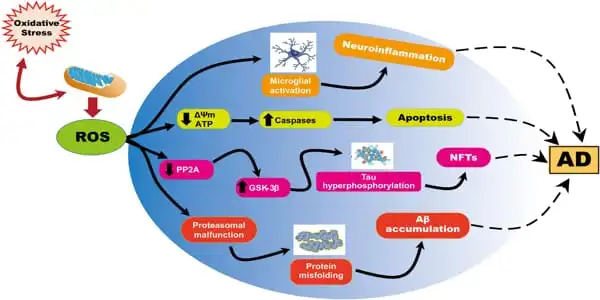
The mitochondrial electron transport chain, which is essential for cellular energy generation, also generates reactive oxygen species (ROS), which assault tissue and cause oxidative damage. This damage can result in mitochondrial malfunction and possibly cell death. Because the brain consumes more oxygen than other organs, it is more prone to ROS damage.
According to the research, ROS also induces amyloid- β (Aβ) accumulation, which signals the development of Alzheimer’s disease (AD), a serious irreversible neurological condition. Treatments for Alzheimer’s disease do not halt the disease’s progression, necessitating the discovery of new medicines.
Mitochondrial activity and cognitive function may be preserved if mitochondria are protected from ROS. Future study should focus on establishing diagnostic markers to detect early brain changes, as well as investigating substances with high antioxidant activity in mitochondria.
Prof. Koji Fukui
A previous study discovered that oxidation levels were significantly higher in elderly rats with vitamin E deficiency than in younger animals. Furthermore, ROS produced by mitochondrial oxidation may cause brain cell damage, revealing a close relationship between Alzheimer’s disease and mitochondrial malfunction. To further this understanding, the same group of scientists has recently shown that the progression of Alzheimer’s disease is tightly linked to oxidative brain damage.
The study, conducted by Prof. Koji Fukui and his colleagues Mr Naoki Yoshida, Mr Yugo Kato, and Prof. Hirokatsu Takatsu, was published in Biomedicines recently. “We demonstrated that oxidation severely damaged the mitochondria, resulting in cognitive impairment,” adds Prof. Fukui, the study’s corresponding author.
The researchers employed three sets of Alzheimer’s disease mice, ages 3, 6, and 20, as well as healthy controls. The mice were tested in two well-known trials to assess their cognitive and coordination abilities: the Morris water maze and the Rota-rod test. They noticed that the Alzheimer’s disease mice took longer to complete their maze tasks but did not slow down. The 6- and 20-month-old AD mice stayed on the rod for longer in the Rota-rod test, but the age-matched control mice dropped faster.
“The difference in fall time could be related to the weight difference between the two groups,” Prof. Fukui notes, “since the control mice were bigger than the AD mice.” These results suggested that AD mice were cognitively impaired but did not have any coordination issues.
To identify which AD-related proteins were responsible for such cognitive impairment, the authors collected tissue samples from various parts of the brain from both groups of mice and assessed the levels of oxidative markers in the samples. First, they found that AD mice had higher levels of Aβ, with a gradual increase observed with age. To their surprise, the AD-related protein Aβ1-42 was significantly higher in the hippocampus than in other parts of the brain. However, they did not find any alterations in the levels of the tau protein, which is another marker that accumulates in AD pathology. Overall, it was confirmed that Aβ1-42 aggregation in the hippocampus caused cognitive impairment in AD mice.
The researchers also speculated that ROS-induced mitochondrial damage was linked to neuron survival. To test this idea, they measured the levels of several critical mitochondrial oxidative enzymes, including the anti-aging nicotinamide-nucleotide adenylyltransferase (NMNAT)-3. While NMNAT-3 levels were shown to be lower in AD mice, levels of 3-NT (3-nitrotyrosine), a sign of greater oxidation, increased with age. “With decreased levels of NMNAT-3 and increased levels of 3-NT, it is clear that oxidation promotes mitochondrial dysfunction, which eventually leads to cognitive dysfunction,” Prof. Fukui says.
The researchers are upbeat about the possible ramifications of their findings, particularly in terms of increasing the intake of antioxidant chemicals that can assist our bodies combat ROS. Indeed, many natural antioxidants, such as vitamins E and C, can be obtained from diet. Prof. Fukui finishes by speculating: “Mitochondrial activity and cognitive function may be preserved if mitochondria are protected from ROS. Future study should focus on establishing diagnostic markers to detect early brain changes, as well as investigating substances with high antioxidant activity in mitochondria.”