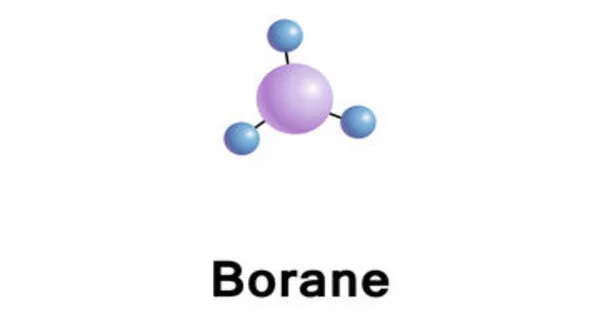
Trihydridoboron, also known as borane, is an unstable and highly reactive molecule with the chemical formula BH3. It is an inorganic compound. It is a colorless gas that only persists at elevated temperatures. It is consisting of a single boron atom carrying three hydrogens. It is a member of boranes and a mononuclear parent hydride.
Boranes are widely used in organic synthesis for hydroboration, which is characterized by addition of BH3 across alkenes to trialkylboranes.
Properties
- Chemical formula: BH3
- Molar mass: 13.83 g·mol−1
- Appearance: colourless gas
- Conjugate acid: Boronium
Preparation
The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser-ablated atomic boron with hydrogen.
Structure
BH3 is a trigonal planar molecule with D3h symmetry. The experimentally determined B–H bond length is 119 pm.
In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction:
BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I)
2 BH3 → B2H6
The standard enthalpy of dimerization of BH3 is estimated to be −170 kJ mol−1. The boron atom in BH3 has 6 valence electrons. Consequently it is a strong Lewis acid and reacts with any Lewis base (‘L’ in equation below) to form an adduct:
BH3 + L → L—BH3
in which the base donates its lone pair, forming a dative covalent bond. Such compounds are thermodynamically stable, but may be easily oxidised in air. Solutions containing borane dimethylsulfide and borane–tetrahydrofuran are commercially available; in tetrahydrofuran a stabilising agent is added to prevent the THF from oxidising the borane. A stability sequence for several common adducts of borane, estimated from spectroscopic and thermochemical data, is as follows:
PF3 < CO< Et2O< Me2O< C4H8O < C4H8S < Et2S< Me2S< Py < Me3N< H−
BH3 has some soft acid characteristics as sulfur donors form more stable complexes than do oxygen donors. Aqueous solutions of BH3 are extremely unstable.
BH3 + 3H2O → B(OH)3 + 3 H2