A new study has found that compounds introduced in the early 1990s to replace ozone-depleting chemicals can accumulate other harmful chemicals in the environment indefinitely.Ozone-depleting chemicals, such as chlorofluorocarbons (CFCs), used in older air conditioners after the Montreal Protocol on ozone-depleting substances in 197one.
The protocol was able to preserve the ozone layer and has been hailed as the most successful global environmental measure ever taken. However, this new study shows that there were some irrational consequences. The chemicals in question are short-chain perfluoroalkyl carboxylic acids (or SKPFCAs), a class of man-made chemicals used in electronic applications, industrial processing, construction, and air conditioning. These belong to a larger group of perfluoroalkyl substances, known as PFAS or “forever chemicals” because of their persistence, which is associated with multiple health problems, including cancer.
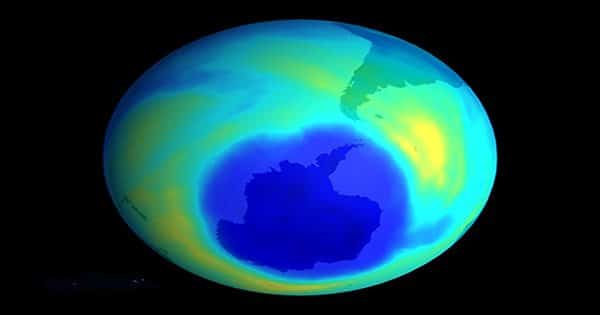
As reported in the Journal of Geophysical Research Letters, researchers at York University and Environment and Climate Change Canada recently discovered the growing presence of scPFCAs in our ecosystem by looking at ice core samples taken from the Arctic.
Cora Young, an assistant professor and environmental chemist at York University, told IFLScience, “Ice cores are useful because they act as capsules of our time and provide a record of pollution. Thus, the only way we can understand these trends is.” “This process will happen everywhere, so we expect pollution worldwide.”
The study did not attempt to understand how the presence of SKPFCAs in the Arctic ice could affect human or environmental health, so it is not clear how these findings were made. Nevertheless, the paper states that the chemicals are “characterized by resistance to environmental degradation and potential adverse effects on human and environmental health.” Since they do not break down in the environment, chemicals work towards their water supply and food, meaning they inevitably end up in human tissues where they accumulate.
Young explained, “The concern for me is that little is known about the potential human and environmental harm of these compounds.” “We know that they gather in plants, they are also accepted by humans, including us also know that they are extremely stable in the environment. Thus, if we discover the road that there is a toxic effect, we will already face their global environment presence.”