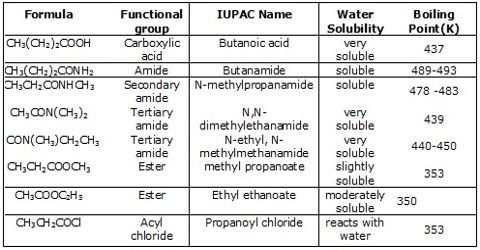
They are weak acids and weaker than mineral acids. Only 0.5% of one molar ethanoic acid is ionized at normal temperature. Only 03 molecules in per thousand, of 1 mole CH3COOH are ionized in per of liter solution.
But, Hydrochloric acid (HCl) or Sulfuric acid (H2SO4) completely dissociate in solution.
If Ka (acid dissociation constant) is high, acidity will by also high.
If pKa is low, acidity will be high.
The value of Ka of CH3COOH is ten trillion times (1010) higher than that of ethanol.
Acid strength decreases as the size of the alkyl group, attached to — COOH group increases. The order is as follows; HCOOH > CH3COOH > CH3CH2COOH
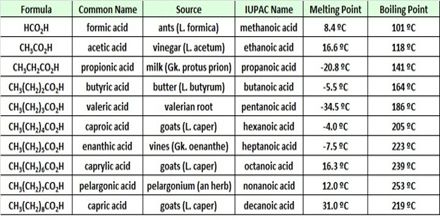
Chloro-ethanoic acid is about times 80 miles (77.78 times) stronger than ethanoic acid. Acid strength increases with an increase in the number of electron – withdrawing the substitute on the n next to the – COOH group, order of acidity is:
H3C-COOH < Cl – CH2 –COOH < Cl2 – CH- COOH < Cl3.C-COOH
The organic acids are weak in the sense that this ionisation is very incomplete. At any one time, most of the acid will be present in the solution as un-ionised molecules. For example, in the case of dilute ethanoic acid, the solution contains about 99% of ethanoic acid molecules – at any instant, only about 1% have actually ionised. The position of equilibrium therefore lies well to the left.