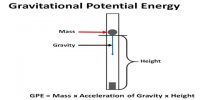
Adiabatic expansion of Carnot Cycle
If the cylinder is taken from the source and is placed on the insulting stand and the piston is moved further so that the volume of the gas changes from V2 to V3 and the pressure changes from P2 to P3. This adiabatic expansion is represented by BC. Since the gas is thermally insulated from all sides no heat can be gained from the surroundings. The temperature of the gas falls from T1 to T2.
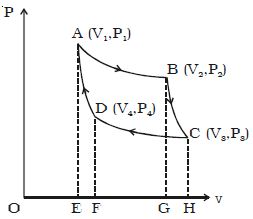
Let W2 be the work done by the gas in expanding adiabatically.
W2 = v3∫v2 PdV = R/(γ-1).(T1-T2) = Area BCHGB