Adsorption from Solution
When a solid is placed into a solution adsorption takes place on the surface of the solid for the same reasons which cause adsorption in the gas-solid system. The aim of this experiment is to study the adsorption of a dye solution by an adsorbent solid such as zeolite, silica gel, activated carbon and then test the validity of certain adsorption isotherm. There is, however, the presence of the solvent which may modify the adsorption. If the solute is a strong electrolyte, the cation and the anion are not likely to be equally adsorbed, particularly at low concentration. In general, one of the ions, depending on the nature of the adsorbent and concentration, is preferentially adsorbed and the particles will acquire an electric charge. The present discussion will be confined to adsorption of non-electrolytes or weak electrolytes.
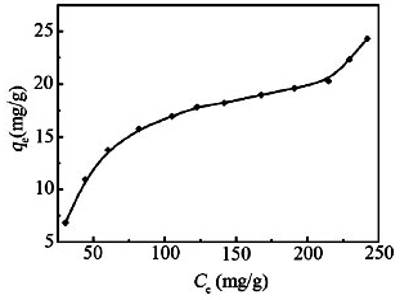
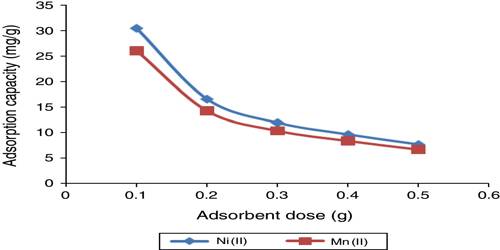
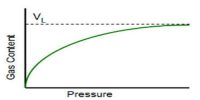
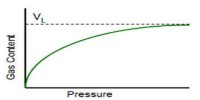
It is observed that at constant temperature the amount of adsorption depends on the concentration of the solute, the higher the concentration the greater the adsorption. The general pattern of the isotherm, as long as physical adsorption takes place, is the same as in the gas-solid system shown in Figure. Deviations occur, as in gas-solid systems, due to multilayer formation or penetration through the surface. As a matter of fact, the basic principles and mechanism in all adsorption processes are the same; the magnitude varies from system to system. In solution, there is the probability of adsorption of the solvent molecules also. If the solute is adsorbed more than the solvent we get the usual positive adsorption and the solute concentration becomes less in the bulk of the solution. But if the solvent is more adsorbed than the solute then the solution becomes more concentrated with respect to the solute.