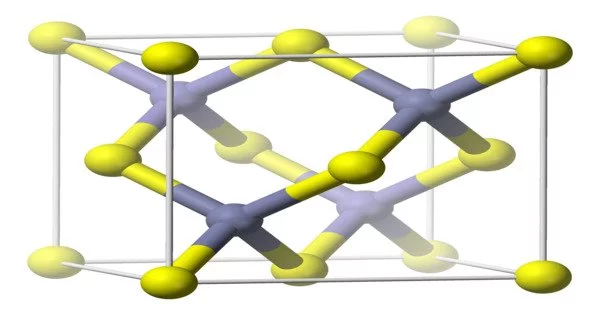
Aluminum arsenide (AlAs) is a binary semiconductor compound made of aluminum and arsenic. It has a zinc blende crystal structure and is a direct bandgap material, making it useful in optoelectronics applications such as light-emitting diodes and laser diodes. It is also used in high-frequency electronics due to its high electron mobility. AlAs is typically grown on a substrate of gallium arsenide (GaAs) using molecular beam epitaxy (MBE) or metalorganic vapor phase epitaxy (MOVPE) techniques.
Aluminium arsenide (AlAs) is a semiconductor with a nearly identical lattice constant to gallium arsenide and a wider band gap than gallium arsenide. (AlAs) can combine with gallium arsenide (GaAs) to form a superlattice, resulting in semiconductor properties. Because GaAs and AlAs have nearly the same lattice constant, the layers have very little induced strain and can be grown to almost any thickness. This enables high electron mobility, HEMT transistors, and other quantum well devices to have extremely high performance.
Properties
- Chemical formula: AlAs
- Molar mass: 101.9031 g/mol
- Appearance: orange crystals
- Density: 3.72 g/cm3
- Melting point: 1,740 °C (3,160 °F; 2,010 K)
- Solubility in water: reacts
- Solubility: reacts in ethanol
- Band gap: 2.12 eV (indirect)
- Electron mobility: 200 cm2/(V·s) (300 K)
- Thermal conductivity: 0.9 W/(cm·K) (300 K)
- Refractive index (nD): 3 (infrared)
- Crystal structure: Zinc Blende
Preparation
Aluminium arsenide is a III-V compound semiconductor material that is useful in the production of optoelectronic devices such as light-emitting diodes. It can be made using well-known techniques such as liquid and vapor-phase epitaxy or melt-growth. However, when exposed to moist air, these aluminum arsenide crystals are generally unstable and produce arsine (AsH3).
Synthesis
Because of the practical difficulties involved, little work on the preparation of aluminum arsenide has been reported. Because of the compound’s high melting point (around 1,700 °C) and the extreme reactivity of aluminium at this temperature, preparation from the melt is difficult. A few workers have created small crystals from the melt, as well as polycrystalline ingots. The best of this material is p-type and has an impurity carrier density of 1019/cm3.
Reactivity
Aluminium arsenide is a stable compound; however, acid, acid fumes, and moisture should be avoided. Hazardous polymerization will not occur. Decomposition of aluminium arsenide produces hazardous arsine gas and arsenic fumes.
Uses
It has a high electron mobility and is used in various electronic and optoelectronic devices such as lasers, light-emitting diodes, and solar cells. It is also used in the production of thin films for optical and electronic applications. AlAs has a direct band gap, which allows it to be used in optoelectronic devices that emit or detect light.
Toxicity
Aluminium arsenide’s chemical, physical, and toxicological properties have not been thoroughly investigated and recorded. Aluminium compounds have numerous commercial applications and are widely used in industry. Many of these materials are chemically active and thus have hazardous toxic and reactive properties.