The earliest known compound of the chemical element argon is argon fluorohydride. It is also known as argon hydrofluorids, a chemical molecule having the formula HArF. (also written ArHF). It is a chemical element argon compound. Argon fluorohydride, which is only stable at very low temperatures, begins to disintegrate as it rises over -246°C. Because of this constraint, it has no applications other than fundamental scientific study.
This first argon compound was discovered by a group of Finnish scientists led by Markku Räsänen. They published their finding of argon fluorohydride in the journal Nature on August 24, 2000.
Properties
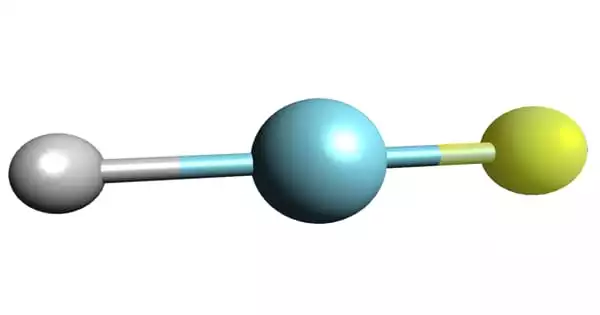
Argon fluorohydride is an inorganic compound with the chemical formula HArF. It is the first known compound of the chemical element argon. It is a compound of the chemical element argon. It is a colorless, odorless gas that is totally inert to other substances.
- Chemical formula: HArF
- Molar mass: 59.954 g/mol
- Appearance: Unknown
- Density: Unknown
- Melting point: −256 °C (−428.8 °F; 17.1 K) (decomposes)
- Solubility in water: Unknown
Discovery
The discovery of this argon compound is attributed to a group of Finnish scientists led by Markku Räsänen. They published their finding of argon fluorohydride on August 24, 2000, in the journal Nature. Despite not being the first, this finding led to the awareness that argon might form weakly bonded compounds.
Argon is collected from liquid air in a cryogenic air separation unit using fractional distillation. When nitrogen gas in the atmosphere is heated with hot calcium or magnesium, a nitride is created, leaving a trace of argon as an impurity.
Synthesis
This substance was created by combining argon and hydrogen fluoride on a caesium iodide surface at 8 K (265 °C) and exposing the combination to UV radiation. As a result, the gases merged. When they examined the substance’s infrared spectra, they discovered that chemical connections had formed, albeit extremely weak ones, as long as the compound was held at temperatures below 256°C. It decomposes into argon and hydrogen fluoride when heated.
The infrared spectrum of the resulting gas combination confirms the presence of chemical bonds, albeit extremely weak ones; consequently, it is argon fluorohydride, not a supermolecule or a mixture of argon and hydrogen fluoride. Its chemical bonds are stable only if the substance is kept at temperatures below 27 K (−246 °C); upon warming, it decomposes into argon and hydrogen fluoride.