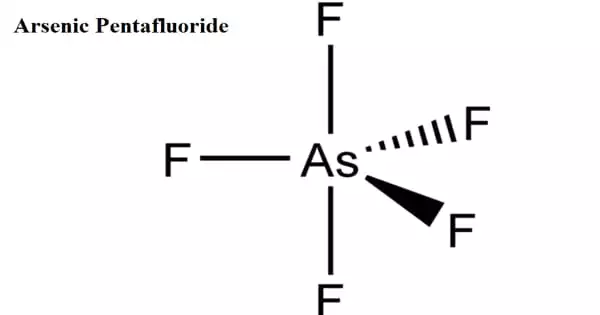
Arsenic pentafluoride is an arsenic and fluorine chemical combination. It is a poisonous, colorless gas. Arsenic has an oxidation state of +5. At room temperature, it is a colorless gas that condenses to a yellow liquid at −53 °C. Although vapor density measurements show some degree of connection, it is a monomeric covalent molecule with a significant degree of coordinating capacity.
Arsenic pentafluoride is a highly corrosive and toxic gas that kills red blood cells and can cause broad systemic damage. It is not flammable, but it is easily hydrolized in the presence of water and humid conditions to generate arsenic and hydrogen fluoride.
Properties
Arsenic pentafluoride is a chemical compound of arsenic and chlorine. It is a colourless gas and has a trigonal bipyramidal structure. In the solid state the axial As−F bond lengths are 171.9 pm and the equatorial 166.8 pm. Its point group is D3h.
- Chemical formula: AsF5
- Molar mass: 169.9136 g mol−1
- Appearance: colorless gas
- Density: 2.138 kg/m3 (g/L)
- Melting point: −79.8 ˚C
- Boiling point: −52.8 ˚C
- Solubility: Ethanol, Dimethylether, Benzene
Pnictogen halide arsenic pentafluoride It is an arsenic fluoride. Arsenic is found free in nature in three metalloidal forms with various crystal shapes (the minerals arsenopyrite and the considerably rarer arsenolamprite and pararsenolamprite), although it is more usually found as a combination with other elements.
Synthesis
Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine:
2As + 5F2 → 2AsF5
It can also be prepared by the reaction of arsenic trifluoride and fluorine:
AsF3 + F2 → AsF5
or the addition of fluorine to arsenic pentoxide or arsenic trioxide.
2As2O5 + 10F2 → 4AsF5 + 5O2
2As2O3 + 10F2 → 4AsF5 + 3O2
Reactions
Arsenic pentafluoride forms halide complexes and is a powerful acceptor as shown by the reaction with sulfur tetrafluoride forming an ionic complex.
AsF5 + SF4 → SF3+ + AsF6−
Fluorine is combined with arsenic metal or arsenic trifluoride to produce it. It is employed in the formation of ionic complexes as a strong Lewis acid fluoride and, in conjunction with Brönsted acids, to generate conjugate superacids. It also forms stable intercalation compounds with graphite that have electrical conductivity comparable to silver.
Safety
Arsenic pentafluoride is a very toxic toxin that primarily poisons liver cells. It has a similar odor to vinyl chloride gas. Because of the potential for significant toxicity, any arsenic compound should be handled with extreme caution.